
CHEMISTRY 3302 April 24, 1998
Instructions:
1) Neither calculators, models, nor notes of any kind are allowed
while taking the exam. The periodic table is, of course, freely
available.
2) Sign the first exam page at the top with your name (please
also print your name to ensure legibility). To be considered
for regrading, the exam must be taken in ink.
3) If a problem is giving you particular difficulty, go on with
other questions and return to it as time allows. The questions
are approximately in order of difficulty.
4) Concise explanations which expand upon your answers are encouraged.
Partial credit will be liberally awarded if it is clear that you
had the right idea, but simply made a small mistake at a later
point in the answer. Don't get carried away, however, as this
will waste your time.
Score
1
2
3
4
Total
I. Molecular Properties (25 points)
Circle the one choice that fits the described property.
Most stable C6H10 isomer

Aromatic system
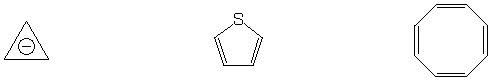
Strongest acid
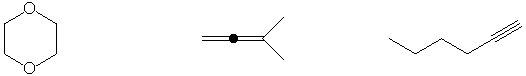
Most reactive dienophile
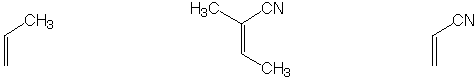
Shows largest peak at M+2 in mass spectrum

II. Kinetics and Thermodynamics (22 points)
Using a potential energy diagram and a specific chemical reaction
as an example, explain the difference between a kinetic and a
thermodynamic product. In particular, address which product is
formed early in a reaction and which dominates after a long time.
The outline of a potential energy diagram is provided with a position
for the reactants (educts) chosen.

III. Chemical Transformations (28 points)
Fill in the boxes with the appropriate educts, reagents, or products.
A normal workup is assumed. Be sure to show stereochemistry where
necessary to distinguish isomers.

IV. Organic Logic and Analysis (25 points)
Shown below is an example of an epoxide being opened by an alkoxide
nucleophile:
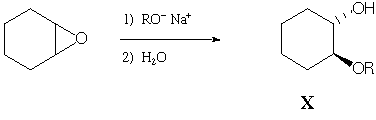
When sodium methoxide is used (R = CH3), product X is formed exclusively. However, when sodium t-butoxide (R = C(CH3)3) is used, a different product, Y, is formed exclusively. Spectral data for Y are provided on the next page (tables of spectral data from Wade are provided on the last page-you may tear this page off if you find it more convenient). Based on these data, propose a structure for Y and show the mechanism for its formation. If you have trouble coming up with a structure, write down what information you do get from analysis of the various spectra-partial credit will be awarded for recognizing key functional groups even if you don't put them together exactly right.
Spectral Data for Y:
IR:
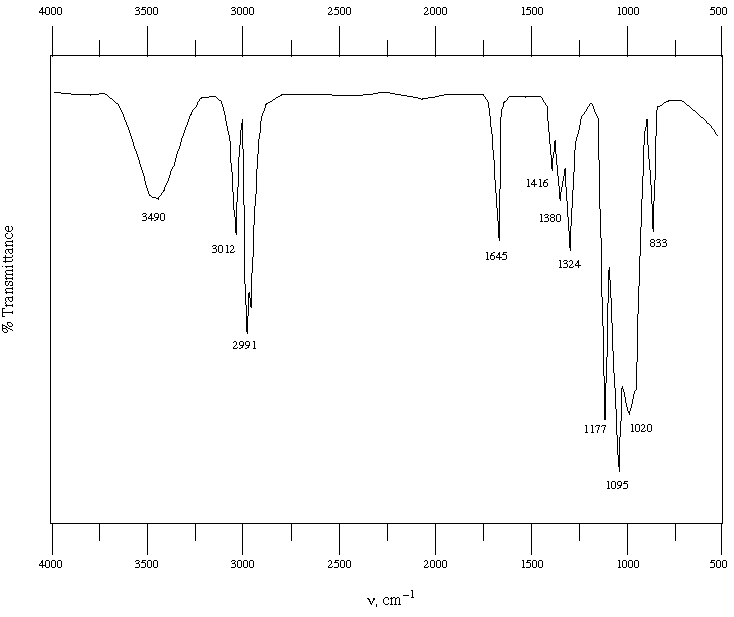
Mass Spec: M+ = 98, large peak at 81, no other
particularly large peaks in the spectrum.
1H NMR d (ppm): 5.8 (doublet of doublets, J = 6.0, 7.5 Hz, 1H)
5.4 (doublet of triplets, J = 6.0, 4.3 Hz, 1H)
4.1 (broad, exchanges with D2O, 1H)
3.9 (multiplet, 1H)
1.8 (multiplet, 2H)
1.4 (multiplet, 4H)