CHEMISTRY 3302 April 24, 1998
I. Molecular Properties (25 points)
Circle the one choice that fits the described property.
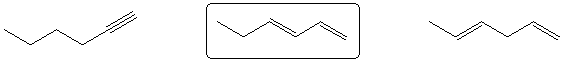
Most stable C6H10 isomer

Aromatic system
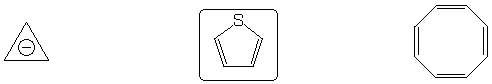
Strongest acid
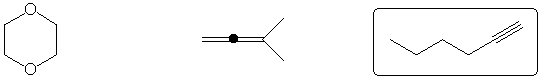
Most reactive dienophile
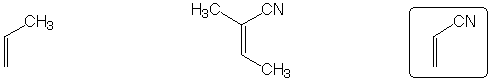
Shows largest peak at M+2 in mass spectrum
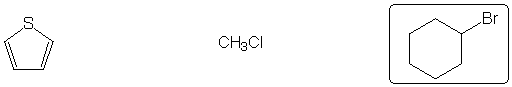
II. Kinetics and Thermodynamics (22 points)
Using a potential energy diagram and a specific chemical reaction
as an example, explain the difference between a kinetic and a
thermodynamic product. In particular, address which product is
formed early in a reaction and which dominates after a long time.
The outline of a potential energy diagram is provided with a position
for the reactants (educts) chosen.
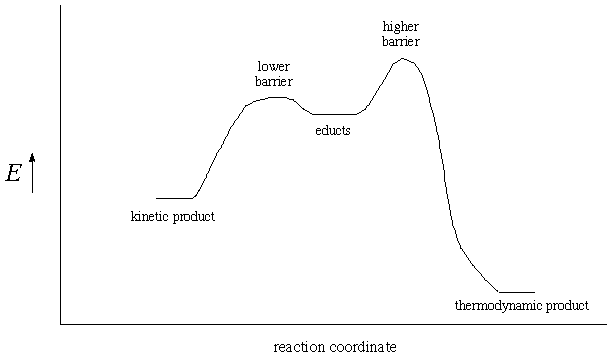
A kinetic product is formed earlier in a reaction because the
activation barrier to form that product is lowest (the Curtin-Hammet
principle says initial product distributions simply reflect the
relative barrier heights). When another product exists at lower
energy, however, eventually everything drains over to this lowest
energy (thermodynamic) product, even though the activation barrier
to form it is higher.
Specific examples include HBr addition to a conjugated diene (kinetic product is 1,2addition and thermodynamic product is 1,4-addition) or Diels-Alder reaction of furan and maleic anhydride, for instance (kinetic product is endo addition, thermodynamic product is exo addition).
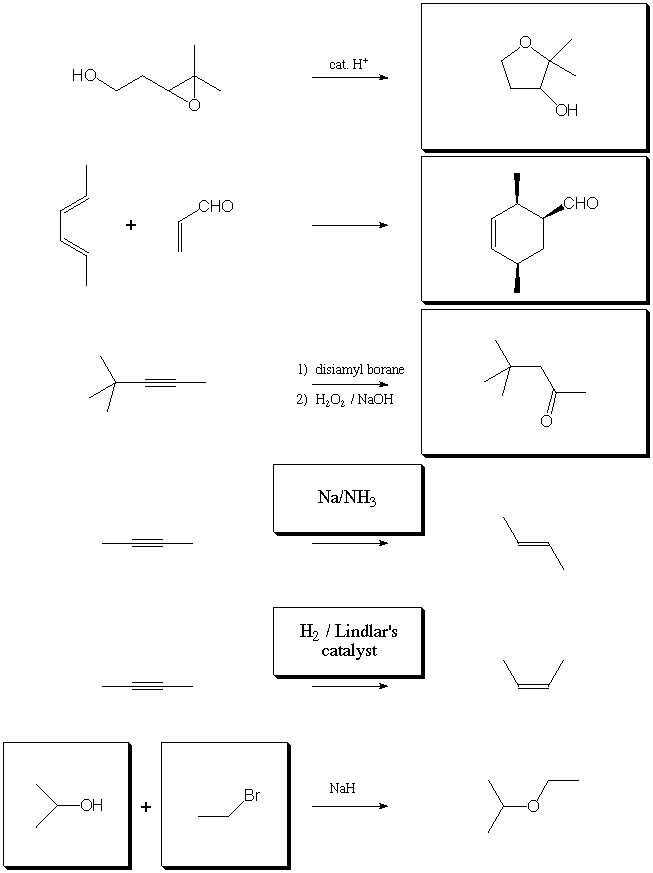
III. Chemical Transformations (28 points)
Fill in the boxes with the appropriate educts, reagents, or products.
A normal workup is assumed. Be sure to show stereochemistry where
necessary to distinguish isomers.
IV. Organic Logic and Analysis (25 points)
Shown below is an example of an epoxide being opened by an alkoxide
nucleophile:
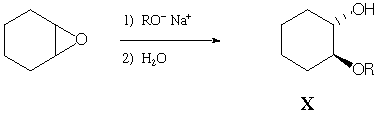
When sodium methoxide is used (R = CH3), product X
is formed exclusively. However, when sodium t-butoxide
(R = C(CH3)3) is used, a different product,
Y, is formed exclusively. Spectral data for Y are
provided on the next page (tables of spectral data from Wade are
provided on the last page-you may tear this page off if you find
it more convenient). Based on these data, propose a structure
for Y and show the mechanism for its formation. If you
have trouble coming up with a structure, write down what information
you do get from analysis of the various spectra-partial
credit will be awarded for recognizing key functional groups even
if you don't put them together exactly right.
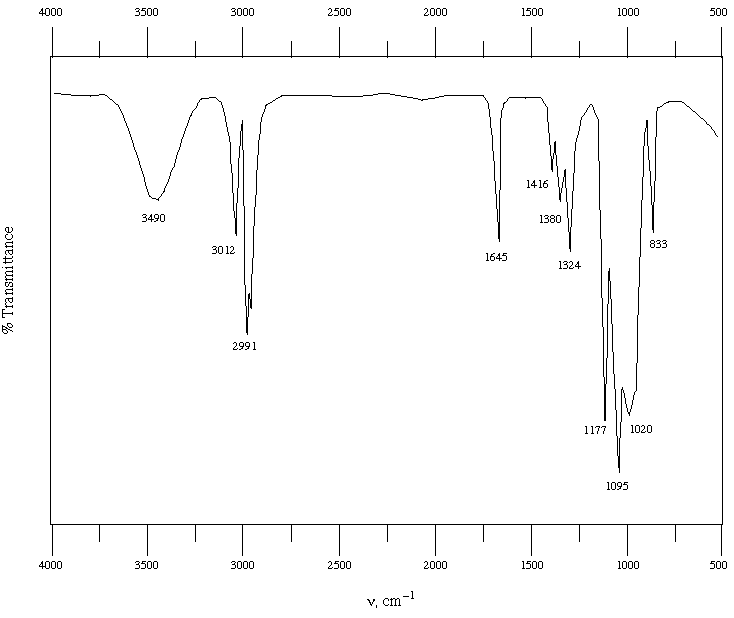
Analysis: Exchangeable proton in NMR and broad peak
at 3500 in IR indicate an OH group. NMR peaks between 5 and 6
ppm and IR absorption at 1645 indicate an alkene with 2 hydrogens
- NMR splitting suggests a cis relationship of hydrogens. IR peak
at 3012 also suggests alkene C-H bonds. Mass spec shows loss of
fragment weighing 17 (OH group) but not much else, suggesting
ring is conserved. NMR peak at 3.9 suggests OH group is attached
to a carbon bearing 1 H. A structure consistent with all of these
data is a cyclohexene with an alcohol attached (gives right total
mass). Peak at 5.8 is a doublet of doublets, so must couple with
a single H on each side. The correct answer is
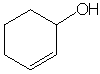
The mechanism for the formation of this product is shown below.
This mechanism is preferred because the large bulk of the t-butoxide
group causes it to act as a base and not a nucleophile.
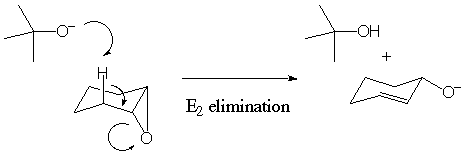
Spectral Data for Y:
IR:
Mass Spec: M+ = 98, large peak at 81, no other
particularly large peaks in the spectrum.
1H NMR d (ppm): 5.8 (doublet of doublets, J = 6.0, 7.5 Hz, 1H)
5.4 (doublet of triplets, J = 6.0, 4.3 Hz, 1H)
4.1 (broad, exchanges with D2O, 1H)
3.9 (multiplet, 1H)
1.8 (multiplet, 2H)
1.4 (multiplet, 4H)