
CHEMISTRY 3302 May 15, 1998
Instructions:
1) Neither calculators nor notes of any kind are allowed while
taking the exam. The periodic table is, of course, freely available.
2) Sign the first exam page at the top with your name (please
also print your name to ensure legibility). To be considered
for regrading, the exam must be taken in ink.
3) If a problem is giving you particular difficulty, go on with
other questions and return to it as time allows. The questions
are approximately in order of difficulty.
4) Concise explanations which expand upon your answers are encouraged.
Partial credit will be liberally awarded if it is clear that you
had the right idea, but simply made a small mistake at a later
point in the answer. Don't get carried away, however, as this
will waste your time.
Score
1
2
3
4
Total
I. Molecular Properties (25 points)
Circle the one choice that fits the described property.
Hydrate found in greatest concentration at equilibrium in
aqueous solution

Strongest acid
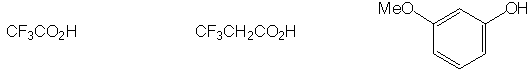
Slowest to react with H2SO4
/ HNO3
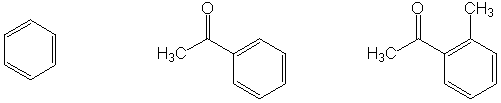
Highest boiling point

Fastest to react with MeO-

II. Chemical Transformations (28 points)
Fill in the boxes with the appropriate educts, reagents, or products.
A normal workup is assumed. Be sure to show stereochemistry if
necessary to distinguish isomers.


III. Organic Logic and Analysis (22 points)
a. Recall that the standard C=O stretching frequency (IR) is 1710 cm-1. Carbonyl groups on strained rings show higher frequency stretches. For instance, cyclopentanone has its C=O stretch at 1745 cm-1. Conjugation, on the other hand, lowers frequencies. Thus, 2cyclopenten-1-one has a C=O stretching frequency of 1715 cm-1. Explain why conjugation lowers the C=O stretching frequency.

b. 3Methyl-2-cyclopenten-1-one has a still lower frequency of 1705 cm-1. Why does methyl substitution further lower the frequency?

c. (Hard) Cyclopentadienone, oddly enough, shows a C=O stretch at 1790 cm-1. Explain this higher frequency stretch. Extra ring strain in cyclopentadienone is not the answer.

IV. Multistep Synthesis (25 points possible)
Complete the synthesis of any one of the target molecules.
The maximum points available per target is shown (harder targets
are worth more points). If you write down more than one synthesis,
indicate which one you want to be graded. For carbon atoms, you
may use only the carbon sources shown below. You may use any
other reagents you like, as long as they do not contribute
a carbon atom to the target. An extra page is attached if you
need more space.
Carbon sources:

Targets:
