I. Molecular Properties (25 points)
Circle the one choice that fits the described property.
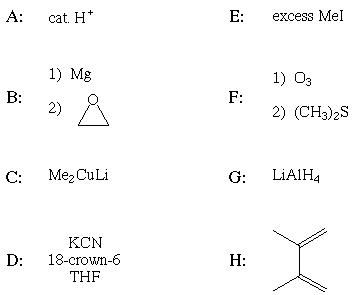
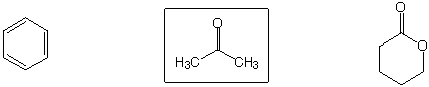
Least reactive to nucleophilic substitution by MeO-

Strongest base
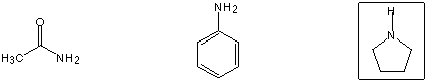
Best acceptor for Michael (1,4) addition
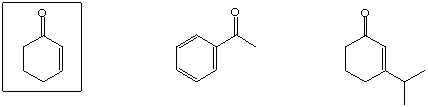
Highest boiling point
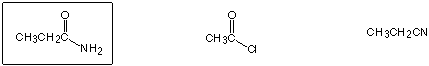
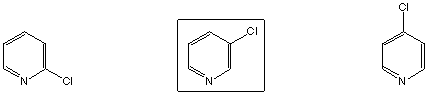
Strongest acid
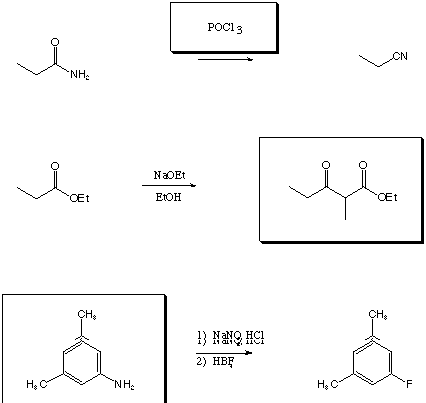
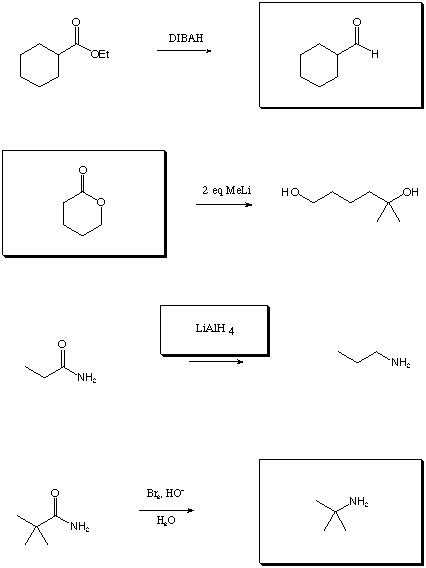
II. Chemical Transformations (28 points)
Fill in the boxes with the appropriate educts, reagents, or products.
A normal workup is assumed. Be sure to show stereochemistry if
necessary to distinguish isomers.
III. Organic Logic and Analysis (20 points)
Consider the following transformation of a g-aminoester
to a lactam:
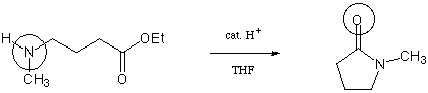
a. Write a step-by-step mechanism for the formation of the lactam.
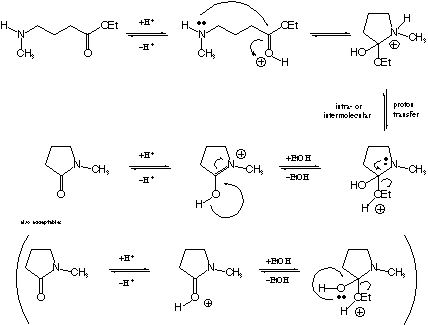
b. On the previous page, circle the most basic atom (i.e., the
site that is most readily protonated by an acid) in the starting
material and in the product. If it is a different atom
in the product than in the starting material, explain why a change
has occurred.
A nitrogen lone pair is nearly always more basic than an oxygen
lone pair of similar hybridization, and the amine has an sp3
lone pair (less s character) while the carbonyl lone pairs are,
by resonance, somewhere between sp2 and sp3
(more s character). Thus, the amine nitrogen is the most basic
atom in the starting material. In the amide, on the other hand,
the amino group donates substantial lone pair density to the carbonyl
via conjugation (or resonance) as illustrated by the below zwitterionic
resonance structure. Thus, in the product, the amide oxygen is
the most basic atom.
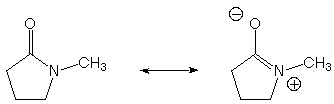
c. This reaction is, in fact, a reversible equilibrium. What
drives this reaction to the right?
The most obvious answer is entropy (two molecules are made from one). In addition, amides tend to be thermochemically more stable than esters (because of the greater importance of the zwitterionic resonance structure for amides than esters) but that might be offset here by slight ring strain.
IV. Multistep Synthesis (27 points possible)
On the next page is a list of 8 reagents (feel free to tear it
off). You will need 6 of these reagents to complete the total
synthesis outlined below (2 are not used). Fill in the reagents
by letter in the boxes over the reaction arrows. The structures
of the starting material, final product, and one intermediate
are provided, as is one set of reagents. Draw the correct structures
for all other intermediates in the boxes provided. Certain
spectral clues are listed under some of the structure boxes. A
normal workup is assumed for all reactions.
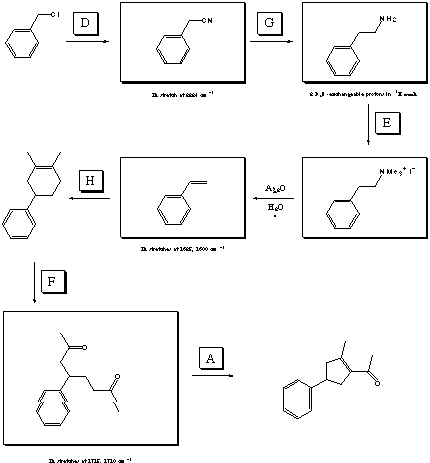
REAGENTS FOR PROBLEM 4: