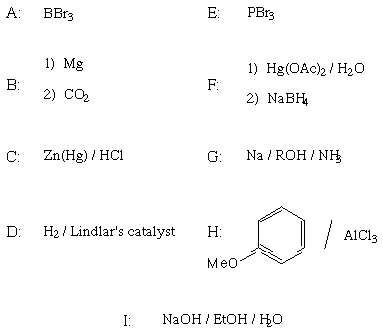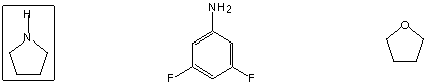
I. Molecular Properties (30 points)
Best hydrogen bond acceptor

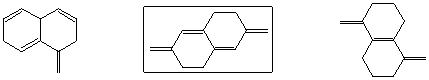
Shows strongest peak at M-15 in mass spectrum
Is reduced by NaBH4
Antiaromatic
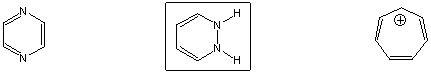
Longest wavelength UV absorption
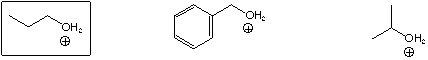
Most stable oxonium ion
II. Problems from Wade (30 points)
Do any two of these (if you work on all three,
indicate which two you want graded).
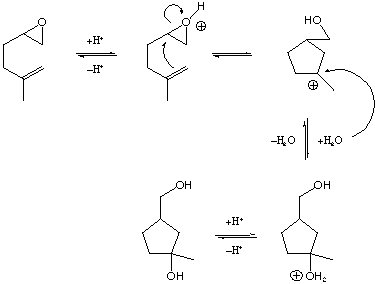
13-32. Show the mechanism for the following reaction:
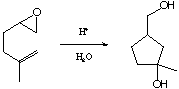
15-29f. What is the product of this reaction (show stereochemistry)?
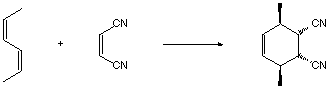
endo addition to the Z,Z-diene leads to the product
shown.
17-54. Triphenylmethanol (below) is insoluble in water, but when
it is treated with concentrated sulfuric acid, it dissolves to
create a bright yellow solution. On dilution of the solution with
more water, the color disappears, and triphenylmethanol precipitates
from solution. Explain this behavior (hint: construct an equilibrium
that generates something likely to be water soluble.
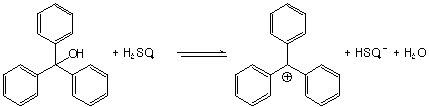
Protonation of the carbinol gives a very unstable oxonium ion (because the triphenylmethyl cation formed after loss of water is so stabilized by resonance). The charged cation dissolves into water. Of course, if water is added, the equilibrium is pushed left, and the neutral triphenylmethanol, which is not water soluble, comes crashing out.
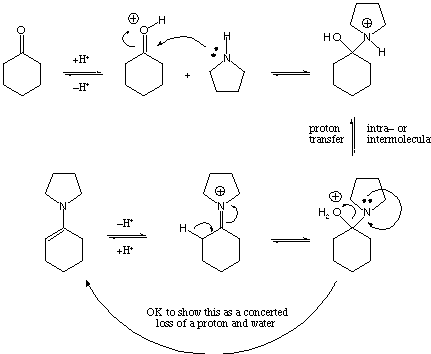
III. Mechanistic Organic Chemistry (20 points)
Show the mechanism for the following reaction:
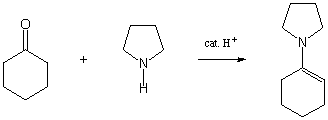
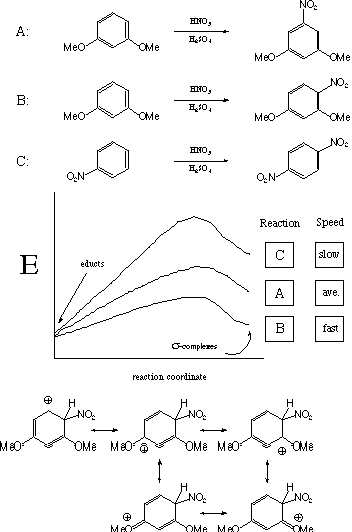
IV. Kinetics/Thermodynamics (30 points)
Label the three potential energy curves with their corresponding
reactions (A, B, or C) in the boxes under "Reaction".
In the boxes under "Speed", label the most rapid reaction
"fast", the slowest reaction "slow"
and the remaining reaction "average". Finally,
draw the s-complex for the fastest
reaction and explain why it is so stable.
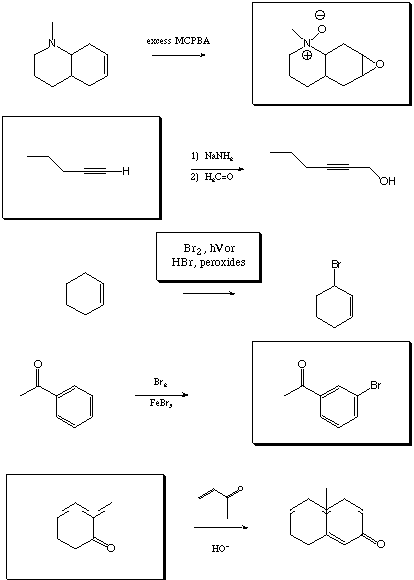
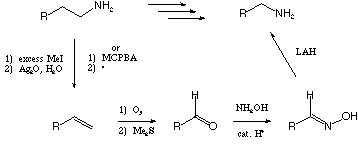
V. Synthetic Transformations 1 (20 points)
Fill in the boxes with the appropriate educts, reagents, or products.
A normal workup is assumed.
 VI. Synthetic Transformations
2 (28 points)
VI. Synthetic Transformations
2 (28 points)
Show the sequence, with reagents, for accomplishing the two chain
lengthening/shortening (homologation/dehomologation) reactions
below.
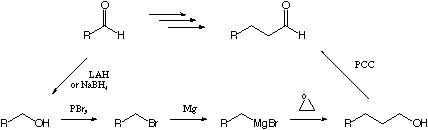
VII. Multistep Synthesis (42 points) (see next page
for instructions)
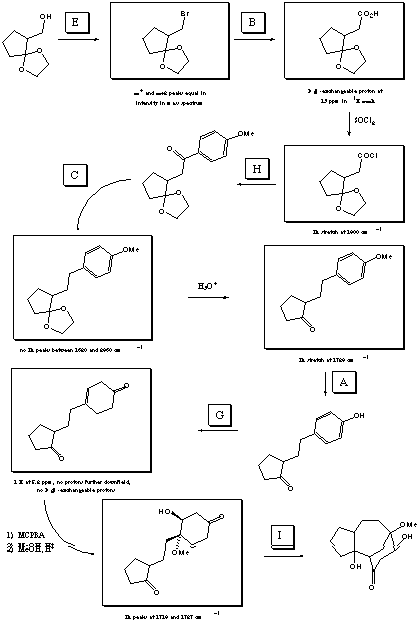
On this page is a list of 9 reagents (you may tear it off). You
will need 7 of these reagents to complete the total synthesis
outlined on the previous page (2 are not used). Fill in the
reagents by letter in the boxes over the reaction arrows.
The structures of the starting material, final product, and two
intermediates are provided, as are three sets of reagents. Draw
the correct structures for all other intermediates . Spectral
clues are listed under all of the structure boxes. A normal
workup is assumed for all reactions.
REAGENTS FOR PROBLEM VII: