Names and ID numbers of group members (please print
legibly):
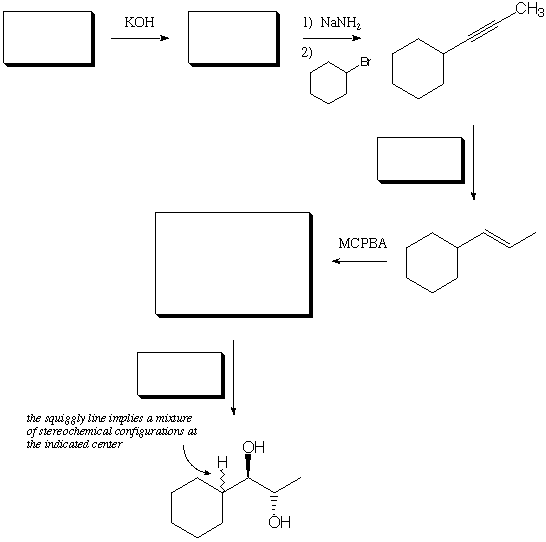
1. Fill in the 5 blanks with educts, reagents, or products, to complete the below series of reactions. (15 points)

2. The following 2 pairs of compounds have identical molecular
weights. Briefly explain how you could use mass spectrometry to
tell them apart. (10 points)
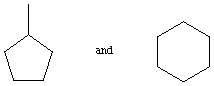
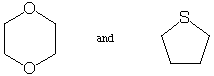
3. Show the step-by step mechanism for the following reaction
(there is no solvent--ethylene glycol is reacted as a "neat"
liquid). You probably want to work it out on a separate sheet
of paper first, and then transfer the correct answer (neatly)
here. (15 points)
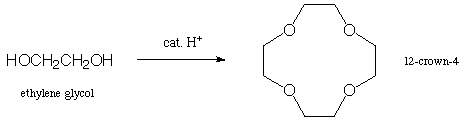
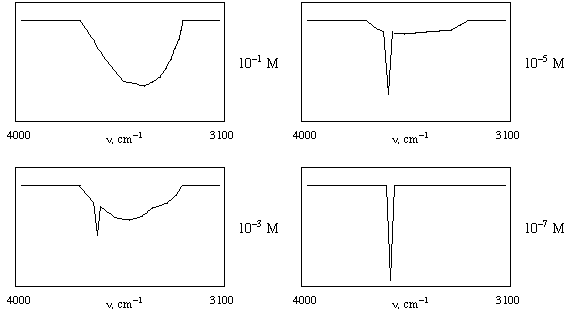
4. Shown below are IR spectra in the 3100-4000 cm-1
region for CH3CH2CH2OH
at 4 different concentrations in CS2 (carbon
disulfide) solvent. Explain the changes in the spectra as the
concentration changes. Note that the C-H stretches do not change,
and they do not appear in these spectra since they are below 3100
cm-1. CS2 solvent also
has no absorptions in the pictured region. [Hint: if you could
take the IR spectrum of a single molecule of 1-propanol,
it would look like the spectrum taken at 10-7
M.] (10 points)