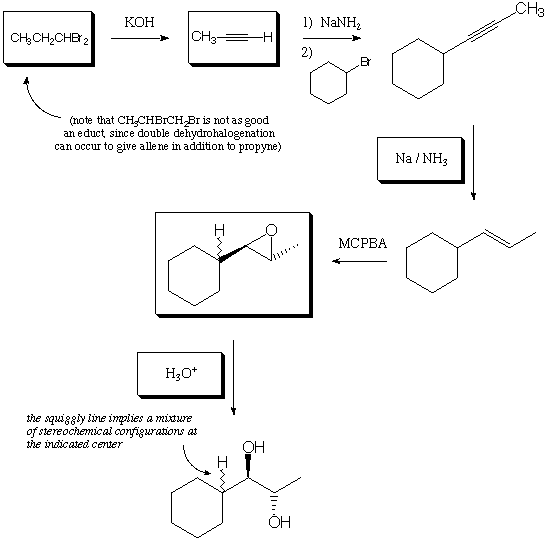
1. Fill in the 5 blanks with educts, reagents, or products, to complete the below series of reactions. (15 points)

2. The following 2 pairs of compounds have identical molecular
weights. Briefly explain how you could use mass spectrometry to
tell them apart. (10 points)
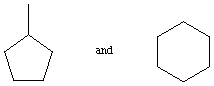
Answer: Methylcyclopentane would be expected to show a strong peak for
loss of CH3 at M-15, while cyclohexane has
no methyl groups to lose.
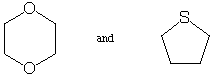
Answer: Sulfur has about a 4% natural abundance of 34S
(the most abundant isotope is 32S), so the
M+2 peak should be 4% of the M+ peak for tetrahydrothiophene
(the molecule on the right). Oxygen, on the other hand, has only
a 0.2% natural abundance of 18O, so the M+2
peak for 1,4-dioxane will be roughly 0.4%, an order of magnitude
smaller relative to M+ than found for the
sulfur case.
3. Show the step-by step mechanism for the following reaction
(there is no solvent--ethylene glycol is reacted as a "neat"
liquid). You probably want to work it out on a separate sheet
of paper first, and then transfer the correct answer (neatly)
here. (15 points)
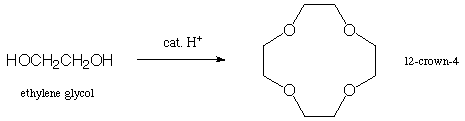
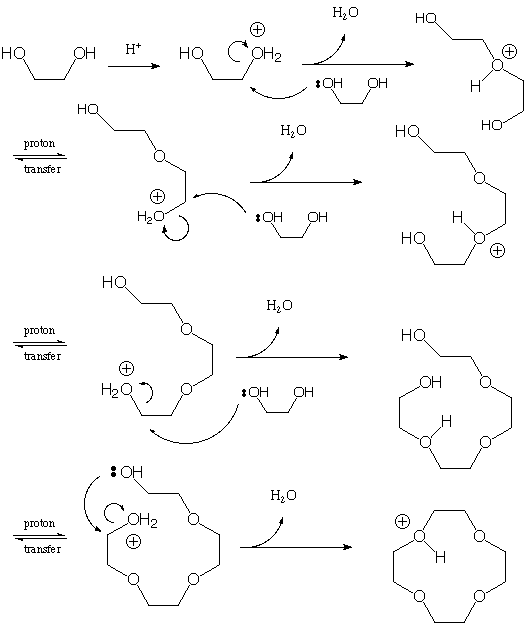
Loss of the (catalytic) proton completes the mechanism. In practice, the reaction is driven by boiling the water out of the system and making those steps irreversible.
4. Shown below are IR spectra in the 3100-4000 cm-1
region for CH3CH2CH2OH
at 4 different concentrations in CS2 (carbon
disulfide) solvent. Explain the changes in the spectra as the
concentration changes. Note that the C-H stretches do not change,
and they do not appear in these spectra since they are below 3100
cm-1. CS2 solvent also
has no absorptions in the pictured region. [Hint: if you could
take the IR spectrum of a single molecule of 1-propanol,
it would look like the spectrum taken at 10-7
M.] (10 points)
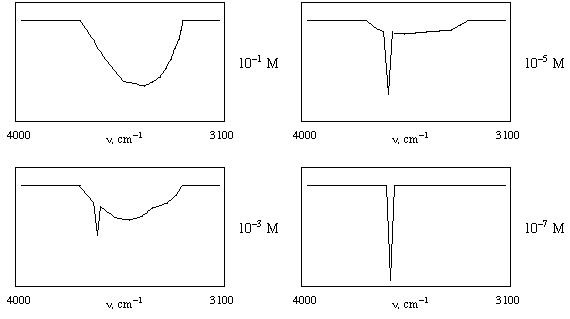
Answer: At high concentrations, the propanol molecules hydrogen bond to one another, and indeed, there are extensive networks of hydrogen bonds that involve many, many molecules. A hydrogen making a hydrogen bond to another molecule cannot make as strong a bond to the molecule it is a part of (a hydrogen bond is like the beginning of a proton transfer, where one would actually fully make a new bond and fully break an old one). This, together with the fact that there are so many different arrangements of hydrogen bonding, leads to hydrogen-bonded O-H stretches being observed over a very broad range of IR frequencies, with this range lower in energy (i.e., lower frequency) than would be found for the non-hydrogen-bonded case. As the concentration is lowered, there are fewer and fewer hydrogen bonding interactions between propanol molecules (they start to become isolated, an effect of entropy) so one sees the "free" O-H stretch grow in. At concentrations so low that no intermolecular hydrogen bonding takes place, only the free O-H stretch is observed; this is, of course, what you would see for a single molecule of propanol if you could measure the IR spectrum for so "dilute" a system.