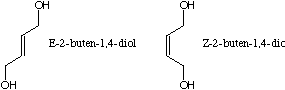
You treat E-2-buten-1,4-diol with two equivalents of PCC
(pyridinium chlorochromate) in dichloromethane solvent. After
1 hr, thin layer chromatography indicates the diol to be completely
reacted. You pour the reaction mixture into a separatory funnel
containing dichloromethane and weak aqueous acid (these two liquids
are immiscible--the intent is for the desired organic compound
to stay in the dichloromethane, while the inorganic salts go into
the water-since pyridine is a mild base, it also goes into the
aqueous acid). After shaking, you discard the aqueous acid, strip
off the dichloromethane under low vacuum, and isolate a clear,
colorless oil--in about the yield you were hoping for! You run
the same reaction with the Z isomer and isolate a different
clear, colorless oil. Strangely enough, when you take a mass spectrum
of the product from the reaction of the E isomer, it has
a mass of 84, but the product from the Z isomer has a mass
of 102 (in each case these are the true molecular ions--no funny
fragmentations going on here).
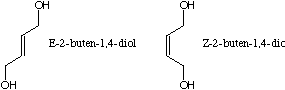
You make up NMR and IR samples of both products, but forget to
label which is which. Your NMR and your IR instruments both break
after running only one sample (you are having a seriously bad
day . . . ) The two spectra you managed to get are attached at
back--you're pretty certain one is for one product, and one is
for the other.
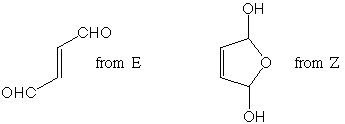
Draw the two products here and label which product comes from which educt:
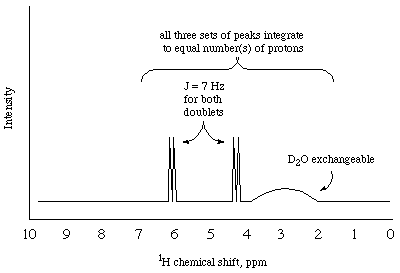
To which of the two products does the provided NMR spectrum belong?
Briefly describe the NMR spectrum expected for the other
product (in particular, the rough chemical shift for each unique
chemical environment and the coupling pattern for each signal--you
may sketch your answer if you can do it neatly).
Provided spectrum is for the product from Z (exchangeable
protons (2), protons on an acetal at approx. 4.2 ppm doublet coupled
to alkene protons at 6.1 -- note only three chemically distinct
environments, 2 protons in each).
The dialdehyde spectrum would be very simple. One doublet between
9 and 10 ppm for the aldehyde protons (doublet from coupling to
vicinal alkene proton) and one doublet near 6 ppm for alkene protons
(same coupling constant since coupling to aldehyde protons). Two
chemical environments, 2 protons each.
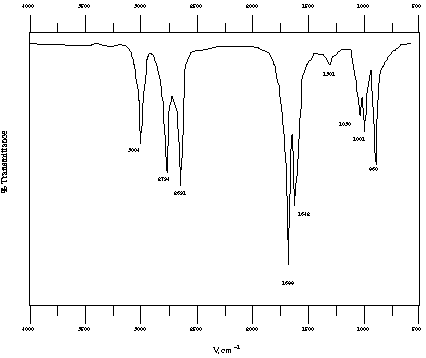
To which of the two products does the provided IR spectrum belong?
Briefly describe the IR spectrum expected for the other
product (i.e., approximately where will peaks occur that provide
information about functional groups? e.g., if a nitrile were the
expected product, you might say "medium intensity peak 2200
cm-1 corresponding to C-N triple bond stretch"-don't
worry about the fingerprint region below 1500 cm-1,
but try to roughly describe every peak above 1500 cm-1--again,
a sketch is OK if it is neat and clearly labeled).
Provided spectrum is for the product from E (the dialdehyde).
Diagnostic stretches include alkene C-H stretch, aldehyde C-H
stretches (2 peaks from coupling with an overtone, remember--see
Wade p. 815), conjugated C=O stretch, and alkene C=C stretch.
The product from Z would be expected to show a broad absorption near 3500 cm-1 for the O-H stretch, alkene and alkane C-H stretches above and below 3000 cm-1, respectively, and an alkene stretch near 1630 cm-1 or so. C-O single bond stretches come in well below 1500 cm-1, so there is not much else to distinguish in this spectrum.
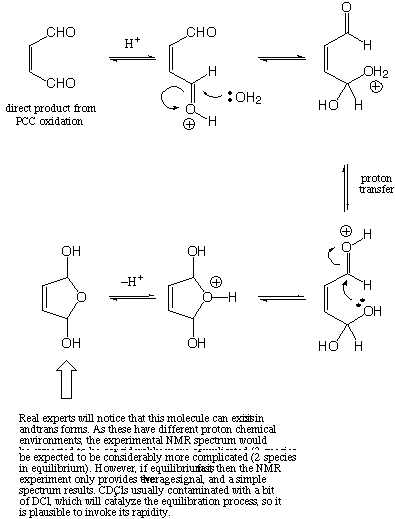
Draw a mechanism for the process whereby an intermediate product
from the Z isomer picks up an "extra" mass of
18 (by comparison to the E isomer) so as to form the isolated
product.
The mistake was washing with acid. Acid catalyzes hydrate formation. For a normal carbonyl, the equilibrium constant lies heavily to the carbonyl side, but being able to form a ring stabilizes the Z hydrate, and it internally ketalizes (i.e., one molecule of water adds in a fashion that bridges both carbonyls).