This exam is to be completed individually. You may use any non-human resource (e.g. your notes, reference books, journals, etc.) to assist you. This exam, along with your critical paper analysis, is due no later than 10 AM on Friday, March 20th. You may turn it in earlier if you wish. Late exams will not be accepted. The completed exam should be turned in to me in 146 Smith, or left in my mailbox (H-18), if you trust that method of delivery.
Please double-space all responses, and use a readable font such as Times 12. If your handwriting is impeccably neat, you may turn in a handwritten exam, although I would prefer that you not.
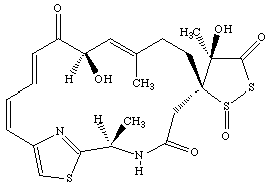
1) This exam consists of a single question in two parts, involving a molecule of current interest. Throughout, assume that you have an allocation of Cray C-90 time which exceeds what you were given in class. Nonetheless, be reasonable1 in your proposals.
Leinamycin belongs to a new class of thiol-activated anti-tumor agents.2,3 It is believed to act through non-specific, single-stranded DNA cleavage, although the exact mechanism is in debate.

a) Clearly, this is a large molecule with a significant degree of conformational flexibility. The x-ray structure4 provides the stereochemistry, which I have reproduced above, but provides only a single structure. The pharmaceutical company you work for (hey, a promotion!) would like to know more about the low-lying conformations that leinamycin can obtain. Describe how you would use any or all of the techniques studied in this class (i.e. classical mechanics/dynamics, semiempirical MO theory, ab initio MO theory and density functional theory) to elucidate the lowest energy conformers of leinamycin, and compare them with the experimentally derived data. Highlight the strengths and weaknesses of any methods you choose. For methods discussed in class that you deem inappropriate for this study, briefly state why. The IR and NMR spectra are known.5 (Limit: 250 - 500 words)
b) Leinamycin is believed to be activated towards DNA cleavage by free thiol.2 Describe how you would study the validity of the proposed mechanism using quantum mechanics. Assume that you can not obtain experimental structural or spectroscopic data for all of the species involved in this pathway. How will you judge the validity of your results? (Limit: 250 - 500 words)
Suggestions:
There is no single correct answer. That is, I am not looking for one magical answer that will earn you full credit. Rather, this exam is designed to test how well you have learned to think critically about computational chemistry. You should construct your answers as if you are writing a brief research proposal. Be very clear about what you plan to do, and why you believe it is the right thing to do. Do not provide me with background information on leinamycin, as you may assume that I have read these papers. You will be graded on the clarity and correctness of your answers, as well as the creativity of your solutions.
1 To judge what is reasonable, simply recall how long it took you to complete the calculations required for your problem sets, then scale accordingly.
2 Behroozi, S. J.; Kim, W.; Gates, K. S. J. Org. Chem. 1995, 60, 3964-3966.
3 Hara, M.; Saitoh, Y.; Nakano, H. Biochemistry 1990, 29, 5676-5681.
4 Hirayama, N.; Matsuzawa, E. S. Chem. Lett. 1993, 1957-1958.
5 Kanda, Y.; Fukuyama, T. J. Am. Chem. Soc. 1993, 115, 8451-8452.