You have 50 minutes to complete this exam. This exam is worth 100 points. Use the back to continue your answers, if necessary.
1) Consider the following energy expression for a hypothetical molecular mechanics force field:
![]()
A B C D E
a) For each of the summed terms in the above expression (A
- E), identify which physical property is being captured.
(15 points)
b) Consider the following molecule, and only the first three terms of the above force field expression for the next three questions (i, ii, and iii).
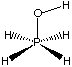
i) Do any of the first three terms require modification of their general form to properly model this molecule? If so, which term(s), and what modification(s) would you make? Do not concern yourself with anharmonicity (i.e. quadratic terms should be considered adequate). (15 points)
ii) What constants would be required in order to evaluate the energy for the H-O-P-H linkage in this molecule? You do not have to provide actual numbers for the constants, simply list them. For example, a calculation on H2 using this force field would require re for H-H bonds. (5 points)
iii) List three reasonable methods which might be used to obtain the constants you have listed for part ii). (5 points)
2) What is the only situation in which it is appropriate to compare the molecular mechanics energy (e.g. the MMX energy) of two molecules? (10 points)
3) The general quantum mechanical Hamiltonian has the form:
What is the central approximation of quantum chemistry which allows us to completely ignore the first term and calculate only once (for any given geometry) the last term in the general Hamiltonian. Provide the name and a brief explanation of the rationale behind the approximation. (15 points)
4) Consider the following z-matrix:
AM1
Chem3D guess
C 0.000000 0 0.000000 0 0.000000 0 0 0 0
C 1.500000 1 0.000000 0 0.000000 0 1 0 0
H 1.086029 1 119.702500 1 0.000000 0 1 2 0
H 1.086014 1 119.702500 1 218.449631 1 1 2 3
C 1.499985 1 59.999008 1 109.225327 1 2 1 3
H 1.086014 1 119.702500 1 250.772934 1 2 1 5
H 1.086044 1 119.702500 1 109.225327 1 2 1 5
H 1.086044 1 119.702500 1 250.769440 1 5 1 2
H 1.086044 1 119.700760 1 109.228821 1 5 1 2
0
a) Sketch the molecule depicted by this z-matrix. (15 points)
b) There are two significant problems with this z-matrix. What are they? (10 points)
c) Explain how you would construct a more appropriate z-matrix. You do not have to write out your new z-matrix. Simply provide me with an indication of how you would do so. (10 points)