Using PC Model, answer the three questions below.
1. Energy minimize the structures of MeCuCN, MeAgCN, and MeAuCN. What are the three heavy atom bond lengths in each of these molecules? Do the structural results seem reasonable? Why or why not? In the breakdown of the MMX energies, you will note that many of the terms are exactly zero--why is that?
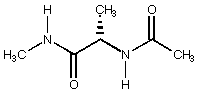
2. When an amino acid is capped at its N-terminus with an acetyl group and its C terminus as an N-methyl amide, it is referred to as a dipeptide; i.e., the alanine dipeptide is drawn below as its fully extended conformer. In a non-polar environment (e.g., in the hydrophobic interior of a protein) the alanine dipeptide prefers to hydrogen bond one amide to the other to create a seven-membered ring. When this happens, the methyl group may either be disposed in a pseudoequatorial or pseudoaxial position (C7eq and C7ax conformations). These dipeptide ring conformers are equivalent to g turns found in protein secondary structure (g turns are possible connections between antiparallel b sheets). What are the relative energies of these three conformers assuming PC Model mimics a hydrophobic environment? Now consider the unnatural amino acid t-leucine (replace the methyl group in alanine with a t-butyl group). What are the relative energies of the three corresponding conformers of t-leucine? Based on your results, would you expect t-leucine to be more or less likely than alanine to be found at the corner of a g turn in an enzyme? (Note: be careful about hydrogen bonding in PC Model -- it's easy to accidentally turn it off!)

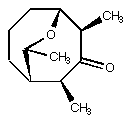
3. The natural product shown below inspires mayflies to stand on their heads. It is thus an important target for total synthesis. You have made it (optically pure, of course). On cooling your NMR tube to -80deg. C, you observe two separate pairs of doublets of quartets in the region of the 1H NMR corresponding to protons alpha to a carbonyl. In one pair, the doublet splittings are 1.1 and 2.7 Hz, respectively, while in the other pair, the doublet splittings are 1.2 and 1.8 Hz, respectively (all quartet splittings are about 7 Hz). Answer the following two questions: (a) why are there two pairs of doublets of quartets? (b) what is the integration ratio of one pair compared to the other (e.g., 50:50, 80:20, etc?) Support your answer with data from PC Model.
Important note on resources: In addition to the computers in 176 Kolthoff, there are platforms in the IT Labs that are running PC Model. In principle, you have access to these machines (assuming you've been assessed the IT computer fee--if you have any trouble, let me know). Locations are EE/CSci 3-170 (M-Th 7 am - 2 am; Fr 7 am - midnight; Sa-Su 10 am - 2 am) and Physics 130 (M-Th 8 am - 10 pm; Fr 8 am - 6 pm; Sa 10 am - 6 pm; Su 4 pm - 10 pm). For output you will need a printer access card, which can be obtained in the lab itself.