Using PC Model, answer the three questions below.
1.The program will happily build polypeptides for you if properly instructed. What is the difference in energy between the a-helical form of Ala5 and it's fully extended (i.e., b-sheet) form? What is the same difference for Gly5? Are your results consistent with the experimental observation that polyalanine forms a helices in water while polyglycine does not? One argument that might be leveled against comparing this calculation to experiment is that it is a gas-phase calculation. Recalculate the energy differences using a dielectric constant of 78.3 (water). How do things change? [Note: One could in principle worry a great deal about whether one should use the zwitterionic form of the polypeptide, whether one should try to optimize the torsions of the amino and carboxyl termini, etc. Don't. Just start from whatever PCModel draws (for goodness sake don't try to draw these by hand -- find the polypeptide construction menu!) and begin your minimizations from there.]
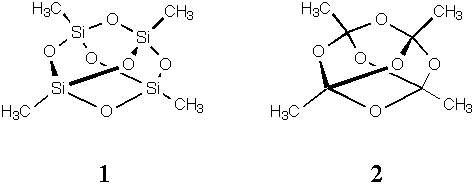
2.Minimize and report the heats of formation for structures 1 and 2. The former is methyl-terminated quartz, if you like, while the latter is a mildly unusual tetra-orthoester. One of these molecules has significantly more bending strain energy than the other. What linkage contributes most to this difference, that is, what angle has a lot more strain in one molecule compared to the analogous angle in the other molecule? You will need to support your answer either by comparing calculated angles to the tabulated equilibrium values in the force field (gotta find those, in that case) or by comparing to ostensibly strain-free molecules having the same linkage. To make life a bit simpler, let's assume all angles have the same force constant (i.e., only displacement from equilibrium matters here).

3.Cyclohexane is well known to have two minimum energy conformations, a chair and a twist-boat. PCModel predicts these to have heats of formation of -29.5 and -24.2 kcal/mol, respectively. This agrees closely with experiment (it was part of the parameterization of the force field, so that need not be surprising). Chair cyclohexane has D3h symmetry, the twist-boat has D2 symmetry--this implies that there is a single unique ring atom in the former, but in the latter there are two kinds of ring atoms. Tabulate the heats of formation for all possible stereoisomers that arise from substituting N, O, Si, P, or S for C (that is, do a complete conformational analysis for piperidine, tetrahydropyran, silacyclohexane, phosphorinane, and pentamethylene sulfide). Note that for the pnictogens, there may be issues associated with stereochemistry at that atom. In addition to the tabulation, comment on any particularly noteworthy trends in the energy differences (limit yourself to 100 words or less on your commentary).
Important note on resources: In addition to the computers in 176 Kolthoff, there are platforms in the IT Labs that are running PC Model. In principle, you have access to these machines (assuming you've been assessed the IT computer fee--if you have any trouble, let me know). Locations are EE/CSci 3-170 (M-Th 7 am - 2 am; Fr 7 am - midnight; Sa-Su 10 am - 2 am) and Physics 130 (M-Th 8 am - 10 pm; Fr 8 am - 6 pm; Sa 10 am - 6 pm; Su 4 pm - 10 pm). For output you will need a printer access card, which can be obtained in the lab itself.