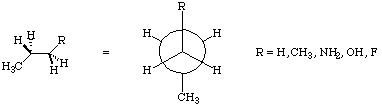
1) Recall that the molecular mechanics torsional potential may be expressed as:
Etorsion = 1/2 V1(1+cosw) + 1/2 V2(1+cos2w) + 1/2 V3(1+cos3w)
where the first term accounts for dipolar interactions, the second for hyperconjugation, and the third for bond-bond repulsions. Now consider the following series:

For each molecule in this series, use the Dihedral Driver under the Analyze menu to vary the dihedral angle (R-C-C-C) to construct a curve with (R-C-C-C) on the x-axis, and MMX energy on the y-axis. Collect enough data points to give a smooth curve which shows all regions of interest. Can you reconcile your graphs with the above equation, i.e. which term(s) seem(s) to dominate in each case? What is the barrier height for rotation in each case? Are there secondary barriers and minima? If so, what are their values? Does all of this seem chemically intuitive. Briefly state why or why not. Why is the MMX energy used for this exercise, rather than the heat of formation? Please attach printouts of your potential energy graphs.
2) Using the drawing tool, draw a hexagon. Use the bond tool (Add_B) to change every second bond from a single bond to a double bond. Click H/AD. You have just made cyclohexa-1,3,5-triene. Minimize this structure and make note of the MMX energy, heat of formation, and C-C bond lengths. Now, select every carbon and choose Piatoms from under the Mark menu. All of the carbons should now have a tilde (~) beside them. Re-minimize, and note the new data. Now, erase that structure, and choose Phen from the Rings menu. Minimize that structure, and note the same data. Which C6H6 structure has the lowest MMX energy (how much)? Which has the lowest heat of formation (how much)? Can you explain your observations? Why do the two types of energies seem to give different results? Repeat the first two steps for cycloocta-1,3,5,7-tetraene, and analyze the data. Can you draw any conclusions about p-delocalizaton, aromatic stabilization or anti-aromatic destabilization? Again, please be brief.
3) Succinic acid [HOOC(CH2)2COOH] is a fairly floppy molecule. Minimize your initial structure and note the geometry and energies. By manipulating the atom positions and re-minimizing, what is the lowest energy form you can find? (I will base part of your points earned for this problem on how low your energy is relative to the structures found by the rest of the class.) Can you locate cyclic and/or hydrogen bonded structures? Report the lowest-energy "linear", cyclic (non-hydrogen bonded) and cyclic (hydrogen bonded) structures (one each is adequate; attach pictures if you wish). Can you rationalize the relative energies of the various minima you have located? Now, under the Options menu, set the dielectric constant to 78.3 (it was 1.5). This will very roughly approximate aqueous solution. Re-minimize your structures. Is the hydrogen bond retained? What is the lowest-energy solvated structure you can find? Compare the heats of formation for your entire ensemble (e = 1.5 and e = 78.3). Do you think it's valid to compare these numbers? Briefly justify your answers.
4) Succinic acid forms a cyclic anhydride by simple heating:
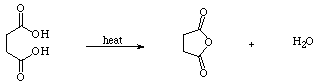
Use PCModel to estimate the barrier for this process, and plot a slice of the potential energy surface (PES) connecting reactant and products, and including any relevant intermediates and/or transition states. Do this with e = 1.5 and e = 78.3. Is the overall reaction endothermic or exothermic? What is the barrier? Do these numbers seem reasonable? (Hint: To plot a valid PES, your equation must always be balanced. For example, do not compare the heat of formation of succinic acid to the heat of formation of succinic anhydride. Instead, compare the heat of formation of succinic acid to the summed heats of formation of succinic anhydride and water.)
Notes on Resources: In addition to the computer lab in 176 Kolthoff (hours: M-Th 9am -9pm, F 9am - 4pm, Sat noon - 4pm), PCModel is installed in the IT labs. You should have access to these machines, assuming you have been assessed the IT computer fee. Locations and hours are: EE/CSci 3-170 (M-Th 7am - 2am, F 7am - midnight, Sat-Sun 10am - 2am) and Physics 130 (M-Th 8am - 10pm, F 8am - 6 pm, Sat 10am - 6 pm, Sun 4pm - 10pm). To print from these machines, you will need a printer access card, which you should be able to obtain in the lab itself.