| Chemistry 8003 | Computational Chemistry | 4 Credits | Winter Quarter 1999 | ( Due 1 / 25 / 97 ) |
Using PC Model, answer any two of the three questions
below.
1. PCModel includes a number of substructure menus designed to
facilitate the building of biopolymers. From the amino acid menu,
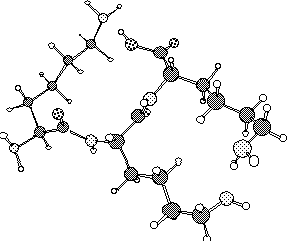
construct the oligopeptide lys-lys-lys. In a biological system
the C terminus would be deprotonated and the N terminus protonated,
but let's not worry about that. The objective of this problem
is to find the lowest energy conformation you can for this tripeptide.
Presumably this will involve some internal hydrogen bonds. Grading
will be based on the range of energies found (lower is better).
When you have found what you think to be the lowest energy structure,
print it out using the print option that includes the energy-try
to orient your structure and choose the display option (e.g.,
ball-and-stick, tube, etc.) so as best to illustrate the actual
conformation. [Note: On the Macintosh, some versions of PCModel
will not send the PostScript file to the printer until you bring
the Finder to the forefront, e.g., by touching the desktop.]

The above structure, which contains 2 internal hydrogen bonds,
has an energy of -11.773 kcal/mol. It is certainly possible that
there are lower energy structures.
2. Use the dihedral driver option in PCModel to construct torsional
potentials about the indicated bonds in the three molecules below.
Construct the potentials over the range of 180 degrees taking
points every 15 degrees (symmetry dictates that only 180 degrees
are unique). Note that PCModel saves the 12 structures to a file,
with their associated energies, so you can open any one of them
to look at its energy and get the breakdown of that energy into
its force field components by doing a single point. By analyzing
the contributions of different force field terms to the total
energy, provide a chemical explanation for any important differences
you perceive between the three potentials.
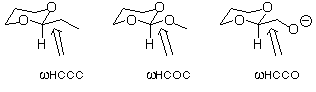
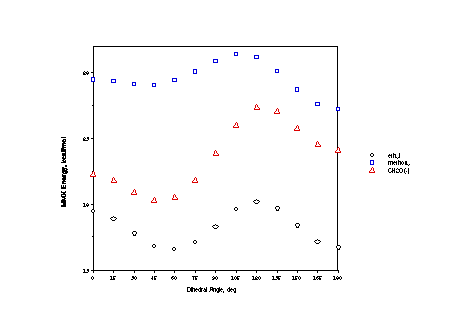
Table 1. Total variation in force field energies and components (kcal/mol).
The variations in bond stretching terms and stretch/bend terms
are much smaller than the total energy variations-bond lengths
do not change much as a function of the dihedral angles, in this
case. Bending terms are 5 to 10 times larger, but still relatively
small compared to the overall energy changes, suggesting that
angle changes are also fairly small as a function of the dihedral
angle.
In the ethyl case, all rotamers have essentially the same electrostatic
term (the atoms in an ethyl group are uncharged in MMX), and the
torsional profile is about 60% in the specific torsional term
and 40% in the van der Waals term. The van der Waals term is about
the same in every case. This may at first glance seem surprising,
since oxygen atoms are "smaller" than alkyl groups,
but remember that C-O bonds are shorter than C-C bonds, so some
atoms are closer together in the oxygenated system.
The torsional term shows a variation of about half the full
energy in each case, however, the periodicity of this term is
very different for the methoxy substituent than for the other
two. As shown on the next page, the methoxy torsional term is
dominated by a two-fold periodic (if plotted over 360 degrees)
component, whereas the other two are dominated by three-fold periodicity.
That is, hyperconjugation dominates the methoxy case (where the
anomeric effect is operative) but sterics dominate the other two
(where it is not).
Finally, in the case of methoxy and methyleneoxide, electrostatics
make a non-trivial contribution to the rotation coordinate (about
30% and 70% of variation, respectively). The variation in this
term tracks the molecular dipole moment closely. Not surprisingly,
oxygen atoms bearing lone pairs, and thus partial or full negative
charges, prefer to minimize their interactions!
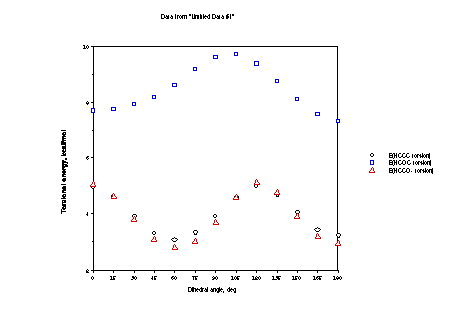
The net effect of the various points made above are that:
1) Ethyl shows typical gauche and trans minima with little
difference in energy between the two.
2) Methoxy shows a deeper minimum for trans (maximal hyperconjugation)
and shows a gauche minimum displaced from 60 in the direction
of 0, and a very low barrier at 0, again because of hyperconjugation.
3) Methyleneoxide shows a deeper gauche minimum than trans
because of the very unfavorable electrostatics for the latter.
Those same electrostatics displace the gauche minimum from 60
in the direction of 0 and dictate a lower barrier at 0 than at
120.
3. Design a problem of your own that uses PCModel to illustrate
some chemically interesting concept. Write down the problem, and
then provide the answer. Note that you do not need to pick something
that PCModel performs well for! However, if you create a problem
where PCModel clearly gives the wrong prediction, provide some
discussion in your answer of why the force field fails to be accurate.
To get more of a feel for typical problems, feel free to drop
by the website and look at first problem sets from previous years.
Grading will be based on quality of the problem and originality.
Obviously, many answers are possible.
Important note on resources: In addition to the computers
in 176 Kolthoff, there are platforms in the IT Labs that are running
PC Model. In principle, you have access to these machines
(assuming you've been assessed the IT computer fee-if you have
any trouble, let me know). Locations are EE/CSci 3-170 (M-Th 7
am - 2 am; Fr 7 am - midnight; Sa-Su 10 am - 2 am) and Physics
130 (M-Th 8 am - 10 pm; Fr 8 am - 6 pm; Sa 10 am - 6 pm; Su 4
pm - 10 pm). For output you will need a printer access card, which
can be obtained in the lab itself.