



|

|

|
Biradical and Zwitterionic Cyclizations of Oxy-substituted Enyne-Allenes
Cramer, C. J.; Kormos, B. L.; Seierstad, M.; Sherer, E. C.; Winget, P.
Org. Lett.
2001, 3, 1881.
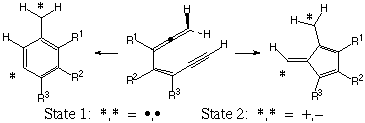
Oxyanion substitution of enyne-allenes causes both Myers-Saito and Schmittel cyclizations to switch their product formation preferences from diradicals to polar, closed-shell singlets. The oxyanion stabilization is larger for the Schmittel products than the Myers-Saito products because the latter must sacrifice aromaticity to maximize interaction. The changing character of the different reaction paths is reflected in their activation energies.
To request a copy of this article, send e-mail to the Research Reports Coordinator at the Minnesota Supercomputer Institute (requests@msi.umn.edu). Please provide a mailing address and specify that you would like UMSI report 2001/109.