



|

|

|
Demonstration of Tunable Reactivity for meta-Benzynes
Nash, J. J.; Nizzi, K. E.; Adeuya, A.; Yurkovich, M. J.; Cramer, C. J.;
Kenttämaa; H. I.
J. Am. Chem. Soc.
in press.
2005, 127, 5760.
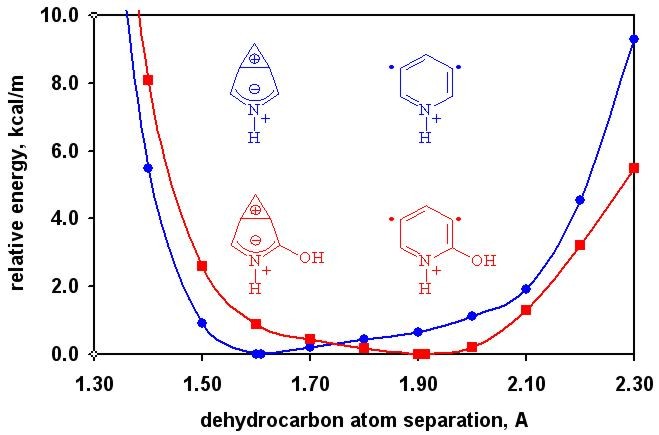
A combined computational and experimental study on the gas-phase structures and reactivities of charged 1,3-didehydroarenes (meta-benzynes) demonstrates that the reactivity of such biradicals can be "tuned" by using appropriate substituents. Substituents that destabilize a specific zwitterionic resonance structure can change the reactivity of the biradical from mildly carbocationic to radical-like. These substituent effects are not the result of changes in the singlet-triplet gaps of the biradicals, but rather changes in the potential energy surfaces for the dehydrocarbon separation.
To request a copy of this article, send e-mail to the Research Reports Coordinator at the Minnesota Supercomputer Institute (requests@msi.umn.edu). Please provide a mailing address and specify that you would like UMSI report 2005/172.