



|

|

|
Aromatic Hydroxylation Reactivity of a Mononuclear Cu(II)-Alkylperoxo Complex
Kunishita, A.; Teraoka, J.; Scanlon, J. D.; Matsumoto, T.; Suzuki, M.;
Cramer, C. J.; Itoh, S.
J. Am. Chem. Soc.
2007, 129, 7248.
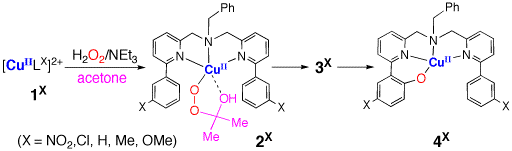
Reaction of copper(II) complex 1X supported by the tridentate bis(pyridylmethyl)amine ligand containing m-substituted phenyl groups at the 6-positions of the pyridine rings (LX) with H2O2 in the presence of triethylamine (NEt3) in acetone generates a copper(II)-alkylperoxo species 2X [2-hydroxy-2-hydroperoxypropane (HHPP) adduct]. The alkylperoxo intermediate 2X undergoes an efficient aromatic ligand hydroxylation reaction, producing a phenolate complex 4X via another intermediate 3X. Kinetic studies on the aromatic hydroxylation process are reported together with spectral characterization of 2X.
To request a copy of this article, send e-mail to the Research Reports Coordinator at the Minnesota Supercomputer Institute (requests@msi.umn.edu). Please provide a mailing address and specify that you would like UMSI report 2007/134.