



|

|

|
Experimental and Theoretical Investigations into the Unusual Regioselectivity of 4,5-, 5,6-, and 6,7-Indole Aryne Cycloadditions
Garr, A. N.; Luo, D.; Brown, N.; Cramer, C. J.; Buszek, K. R.;
VanderVelde, D.
Org. Lett.
in press
(doi:10.1021/ol902415s).
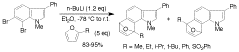
The 6,7-indolyne shows remarkable regioselectivity in its cycloaddition with 2-substituted furans. Electron-donating groups give predominantly the more sterically crowded product, while electron-withdrawing groups display the opposite regioselectivity. By contrast, 4,5- and 5,6-indolynes show no regioselectivity. Optimized electronic structure calculations using the M06-2X density functional and 6-311+G(2df,p) basis set revealed that the 6,7- indolynes are highly polar structures, and that their cycloadditions have substantial electrophilic substitution character that leads to the observed preference for contrasteric products.