Li, H.; Yu, Y.; Ruan, M.; Jiao, F.;Chen, H.; Gao, J.; Weng, Y.; Bao, Y.. Biophysical Journal, 2022, 121, 2233–2250.
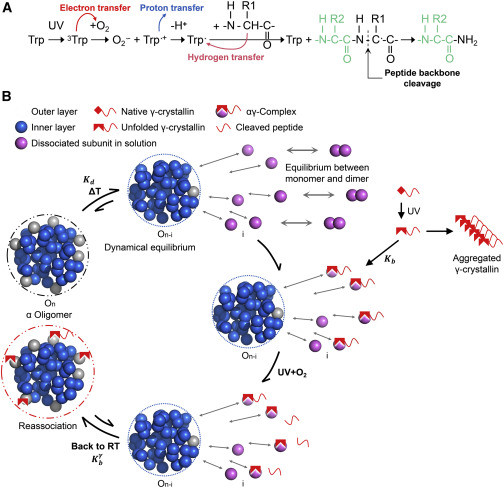
Exposure to solar UV irradiation damages γ-crystallin, leading to cataract formation via aggregation. α-Crystallin, as a small heat shock protein, efficiently suppresses this irreversible aggregation by selectively binding the denatured γ-crystallin monomer. In this study, liquid chromatography tandem mass spectrometry was used to evaluate UV-325 nm irradiation-induced photodamage of human γD-crystallin in the presence of bovine α-crystallin, atomic force microscope (AFM) and dynamic light scattering (DLS) techniques were used to detect the quaternary structure changes of the α-crystallin oligomer, and Fourier transform infrared spectroscopy and temperature-jump nanosecond time-resolved IR absorbance difference spectroscopy were used to probe the secondary structure changes of bovine α-crystallin. We find that the thermal-induced subunit dissociation of the α-crystallin oligomer involves the breaking of hydrogen bonds at the dimeric interface, leading to three different spectral components at varied temperature regions as resolved from temperature-dependent IR spectra. Under UV-325 nm irradiation, unfolded γD-crystallin binds to the dissociated α-crystallin subunit to form an αγ-complex, then follows the reassociation of the αγ-complex to the partially dissociated α-crystallin oligomer. This prevents the aggregation of denatured γD-crystallin. The formation of the γD-bound α-crystallin oligomer is further confirmed by AFM and DLS analysis, which reveals an obvious size expansion in the reassociated αγ-oligomers. In addition, UV-325 nm irradiation causes a peptide bond cleavage of γD-crystallin at Ala158 in the presence of α-crystallin. Our results suggest a very effective protection mechanism for subunits dissociated from α-crystallin oligomers against UV irradiation-induced aggregation of γD-crystallin, at the expense of a loss of a short C-terminal peptide in γD-crystallin.