
 |
|
Recent Research Developments |
|
August 22, 2001
|
|
|
Green Chemistry : Iron-Catalyzed Asymmetric Olefin cis-Dihydroxylation |
|
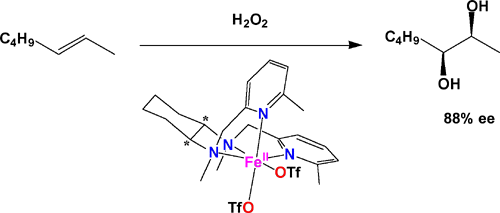
In a communication published in the Journal of the American Chemical Society (J. Am. Chem. Soc. 2001, 123, 6722-6723) and highlighted in a recent C&E News of the Week feature (July 23, 2001), postdoctoral associates Miquel Costas, Adrianne Tipton, and Du-Hwan Jo, graduate student Kui Chen, and Professor Lawrence Que report the first example of an iron catalyst for asymmetric cis-dihydroxylation of olefins. The cis-dihydroxylation of olefins is usually carried out by the heavy metal reagent OsO4, and Sharpless has developed catalytic asymmetric versions of the reagent to overcome inherent problems with Os toxicity. Inspired by the fact that cis-dihydroxylation is carried out in nature by nonheme iron enzymes, Costas et al. have discovered that the complex [Fe(6-Me2-BPMCN)(O3SCF3)2] (6-Me2-BPMCN = N,N'-bis(6-methylpyridyl-2-methyl)-trans-1,2-diaminocyclohexane) can catalyze the cis-dihydroxylation of olefins with H2O2 as oxidant. An enantiomeric excess of as high as 88% can be obtained with trans-2-heptene. This iron complex could thus be considered the first example of a "greener" or more environmentally friendly version of an olefin cis-dihydroxylation catalyst. |
|
|
|
* This page is updated every two weeks.
Next scheduled update: Sept. 5, 2001.
The University of Minnesota is an equal opportunity educator and employer. Copyright 2003 by the Regents of the University of Minnesota. For questions or comments, contact the Chemistry Webmaster.
|