
 | |
Recent Research Developments | |
| Index of Recent Research News |
| April 16, 2003 | |
|
|
|
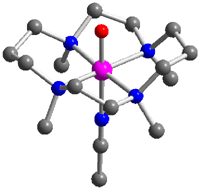
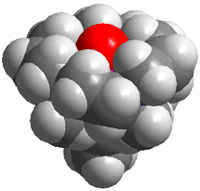
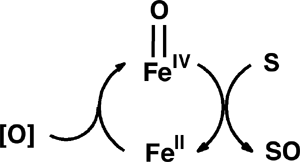
Metabolically important oxidative transformations that are carried out by iron enzymes and O2 are in the focus of bioinorganic chemistry research. The catalytic cycles of mononuclear nonheme iron enzymes are often proposed to involve a high-valent iron-oxo intermediate as key oxidant, but evidence for these species is at best only indirect. Postdoctoral associate Jan-Uwe Rohde and visiting students Mi Hee Lim, M.S., and Jun-Hee In in the group of Professor L. Que have recently succeeded in the generation and isolation of the first examples of such transient species from synthetic iron(II) complexes. As reported in Science (2003, 229, 1037) and Proc. Natl. Acad. Sci. U.S.A. (2003, 100, 3665), mononuclear complexes with a terminal FeIV=O unit have been formed in stoichiometric reactions with a peracid or iodosylbenzene as oxygen donors at low temperature (Figure, bottom). New intermediates are commonly discovered by UV/vis spectroscopy. These novel iron(IV)-oxo molecules, however, exhibit transitions in the vis/NIR region (720-820 nm), the low intensities of which (ε ≈ 300-400 M-1cm-1) apparently precluded their discovery until now. The supporting ligand tetra-N- methylcyclam (TMC) greatly enhances the lifetime of the FeIV=O moiety allowing the crystallization and isolation of [FeIV(O)(TMC)(NCMe)](OTf)2. Its high-resolution crystal structure reveals an Fe-O bond length of 1.646(3) Å, demonstrating that a terminal FeIV=O unit can exist in a nonporphyrin ligand environment (Figure, top). The snapshot of this reactive intermediate provides mechanistic clues into O2 activation cycles, as highlighted in C&EN (2003, 81(7), 13) and in a Science Perspective (2003, 299, 1024). |
|
|
|
|
|
Figure. Molecular structure and space-filling representation of [FeIV(O)(TMC)(NCMe)]2+ (top); generation of [FeIV(O)(TMC)(NCMe)]2+ and [FeIV(O)(TPA)]2+ and subsequent oxygen transfer to substrates (bottom); key to atoms: pink = Fe, blue = N, gray = C, red = O; TMC = 1,4,8,11-tetramethyl-1,4,8,11-tetraazacyclotetradecane, TPA = N,N,N-tris(2-pyridylmethyl)amine, S = PPh3, PhSMe, olefin. The nearly quantitatively formed complexes [FeIV(O)(TMC)(NCMe)]2+ and [FeIV(O)(TPA)]2+ have been extensively characterized by electrospray mass spectrometry, Mössbauer and X-ray Absorption Spectroscopy. They also exhibit considerable differences not only with regard to their stability properties, but also in their oxygen-atom transfer capabilities towards substrates. These observations emphasize the important role ligand structure can play in modulating stability and reactivity of the iron(IV)-oxo unit. These projects further involve substantial contributions from graduate students William W. Brennessel (X-ray crystallography), Michael R. Bukowski (electrospray mass spectrometry and vibrational spectroscopy), and Audria Stubna (Mössbauer spectroscopy at Carnegie Mellon University). This project is a collaboration among three groups in three universities: the Que group at the University of Minnesota, the group of Professor Wonwoo Nam at Ewha Womans University in Seoul, Korea, and the group of Professor Eckard Münck at Carnegie Mellon University in Pittsburgh, PA. |
|
| * This page is updated every two weeks. Next scheduled update: Apr. 30, 2003. |
|
|
The University of Minnesota is an equal opportunity
educator and employer.
Copyright 2003 by the Regents of the University
of Minnesota.For questions or comments, contact the Chemistry
Webmaster or read the University's Online Privacy Statement. |
|