
 | |
Recent Research Developments | |
| Index of Recent Research News |
| August 20, 2003 | |
|
|
|
|
The most selective chemical sensors recognize the analytes that they are designed to sense by forming a complex with a host compound. In the case of ionic analytes, these compounds are referred to as ionophores. Their synthesis and optimum use is of primary importance to the development of new chemical sensors for a multitude of applications, such as in environmental and clinical analysis or industrial process control. As discussed in two citation classics by Philippe Buhlmann and his collaborators at the Swiss Federal Institute of Technology in Zurich Switzerland and Auburn University in Auburn AL (Chem. Rev. 1997, 97, 3083-3132; Chem. Rev. 1998, 98, 1593-1687), ionophore-based potentiometric sensors are among the most successful chemical sensors. |
|
|
|
|
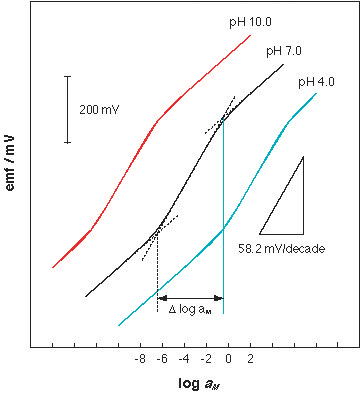
Every introductory textbook of analytical chemistry explains that a potentiometric sensor should respond to a tenfold increase in the activity of dication with a 29 mV increase of the measured electrical potential. However, since the late 1980s, various researchers have reported on ionophore-based sensor responses that are twice as large as expected. Pointing out the difference between classical responses, as described by the famous electrochemist Walther Nernst, these unusual responses have been described as being "twice-Nernstian". Investigations of several ionophores with high promise for clinical applications were continued for years but stalled because their twice-Nernstian responses were not understood. |
|
|
|
|
Philippe Buhlmann and his former advisee Shigeru Amemyia (since 2002 on the faculty of the University of Pittsburgh) report now on a general model that explains twice-Nernstian responses and predicts new oddities, such as half-Nernstian or triple-Nernstian responses (Anal. Chem. 2003, 75,3329-3339). Their approach is an extension of their earlier work with ion exchangers (Electroanalysis 1999, 11, 687-693), ionophores with antibiotic properties (Anal. Chem. 1998, 70, 445-454), and metalloporphyrins (Anal. Chem. 2000, 72, 5766-5773). The new, general model quantitatively explains how the response of these sensors depends on the stoichiometry and stability of the complexes of the analyte and ionophore, and how the response may be affected by other ions such as hydrogen or hydroxide ions. Like its two predecessor papers, which have guided already several research groups in their sensor design, this contribution promises to be a new valuable tool in the preparation of ever more selective and sensitive chemical sensors. |
|
| * This page is updated every two weeks. Next scheduled update: Sept 3, 2003. |
|
|
The University of Minnesota is an equal opportunity
educator and employer.
Copyright 2003 by the Regents of the University
of Minnesota.For questions or comments, contact the Chemistry
Webmaster or read the University's Online Privacy Statement. |