
 | |
Recent Research Developments | |
| Index of Recent Research News |
| June 11, 2003 | |
|
|
|
|
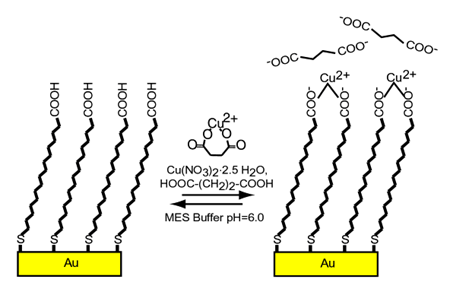
The binding of metal ions to monolayer assemblies is important in many disciplines. The ability of surface functional groups to selectively bind certain metal ions has shown their utility in chemical sensing and environmental clean-up. Metal ions bound to surface groups have been used for the immobilization of protein molecules for purification, sensing, and two-dimensional crystallization. In the so-called "molecular beaker epitaxy", metal ion binding to surface phosphate groups is used to form multilayer films. Similar strategies have been extended to other ligands, such as pyridine, carboxylate, and sulfonic acid. In the examples listed above, the thermodynamics of metal ions binding to surface ligand groups is a key issue, but this issue has not been addressed in past studies. It should be noted that one may not simply extrapolate solution phase metal coordination chemistry to surfaces. One distinguishing factor related to the coordination of metal ions to surfaces is that the surface functional groups can be arranged in a two-dimensional array. Recent experiments by Graduate Student Ryan Major and Chemistry Professor Xiaoyang Zhu explore how this two-dimensional arrangement lead to an inherent chelate effect, which can be call the ãsurface chelate effectä. This work has been accepted for publication as a communication to the Journal of the American Chemical Society. The presence of a surface chelate effect is established in the model system of Cu2+ adsorption on a self-assembled monolayer of 16-mercaptohexadecanoic acid (MHA) on Au. The formation constant of Cu2+ with the MHA surface was found to be 119 ±3.2 times greater than Cu2+ with succinic acid (HOOC-(CH2)2-COOH), and 213 ± 4.0 times greater than Cu2+ with glutaric acid (HOOC-(CH2)3-COOH) in aqueous solutions. Both of these molecules are known to chelate to metal ions forming 7 and 8-membered rings. The chelate effect refers to the greater stability of metal complexes of multidentate ligands than those of unidentate ligands. Consider the coordination of two carboxylic groups to one Cu2+. In the case of acetic acid, this involves two bi-molecular reactions steps. However, in the case of a bi-dentate ligand, such as succinic or glutaric acid, the coordination of the second ligand is a uni-molecular reaction. Thus, there is tremendous statistical advantage for the bi-dentate in the formation of the second reaction step. Energetic gain or penalty associated with the formation of the cyclic metal-bidentate bond is usually of less significance than the statistical gain. Indeed, the reaction rate for the second coordination bond formation and, thus, the overall equilibrium constant involving bidentate, can be orders of magnitude higher than that of uni-dentate. Such a statistical advantage is further enhanced when one considers the binding of the Cu2+ to a two-dimensional array of surface ligands. After the coordination to one surface carboxylic group, there is a great propensity for the metal ion to bind to one of the six neighboring carboxylic groups within an ordered monolayer. The surface chelate effect demonstrated here is of general significance to adsorption on molecular surfaces and should depend strongly on chemical functionality and monolayer structure. This effect can be advantageously incorporated into the design of monolayer-based technologies, such as those discussed in the beginning. | |
 |
|
| * This page is updated every two weeks. Next scheduled update: June 25, 2003. |
|
|
The University of Minnesota is an equal opportunity
educator and employer.
Copyright 2003 by the Regents of the University
of Minnesota.For questions or comments, contact the Chemistry
Webmaster or read the University's Online Privacy Statement. |