
 | |
Recent Research Developments | |
| Index of Recent Research News |
| October 29, 2003 |
| |
| The metal catalyzed ring-opening polymerization of lactide (LA), a renewable resource, is an efficient way to produce a biodegradable and recyclable plastic, polylactide (PLA). The commercial production of PLA for applications in medicine, packaging, and fiber technology relies on this important catalytic transformation. Diverse metal alkoxide complexes exhibit varying degrees of effectiveness for the polymerization of LA (and related cyclic esters), as reflected by differing rates and levels of polymerization control and stereoselectivity. Notably lacking is fundamental mechanistic knowledge to help understand these differences and facilitate next-generation catalyst design. In a recently published article1, Prof. William Tolman, Prof. Marc Hillmyer, and coworkers reported the preparation, structural characterization, and detailed lactide polymerization behavior of a new Zn(II) alkoxide complex, (L1ZnOEt)2 (L1 = 2,4-di-tert-butyl-6-{[(2-dimethylaminoethyl)-methylamino]methyl}phenolate). While an X-ray crystal structure revealed the complex to be dimeric in the solid state (Figure), nuclear magnetic resonance and mass spectrometric analyses showed that the monomeric form L1ZnOEt predominates in solution. The polymerization of lactide using this complex proceeded with good molecular weight control and gave relatively narrow molecular weight distribution polylactide, even at catalyst loadings of <0.1 % that yielded Mn as high as 130 kg mol-1. The effect of impurities on the molecular weight of the product polymers was accounted for using a simple model. Detailed kinetic studies of the polymerization reaction enabled integral and non-integral orders in L1ZnOEt to be distinguished and the empirical rate law to be elucidated, d[LA]/dt = k p[L1ZnOEt][LA]. These studies also showed that L1ZnOEt polymerizes lactide at a rate faster than any other Zn-containing system reported previously. This work provides important mechanistic information pertaining to the polymerization of lactide and other important cyclic esters by discrete metal alkoxide complexes. 1Williams, C. K.; Breyfogle, L. E.; Choi, S. K.; Nam, W.; Young, V. G., Jr.; Hillmyer, M. A.; Tolman, W. B. J. Am. Chem. Soc. 2003, 125, 11350-11359. |
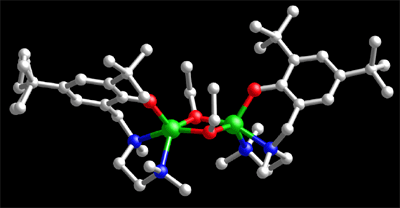
X-ray crystal structure of the new lactide polymerization catalyst, (L1ZnOEt)2. Zn atoms are green, nitrogen atoms blue, oxygen atoms red, and carbon atoms gray. |
| * This page is updated every two weeks. Next scheduled update: Nov. 5, 2003. |
|
The University of Minnesota is an equal opportunity
educator and employer.
Copyright 2003 by the Regents of the University
of Minnesota.For questions or comments, contact the Chemistry
Webmaster or read the University's Online Privacy Statement. |