
Recent Research Developments
Nanoparticles inherently maximize surface-to-volume ratio and, as a result, researchers in surface science and surface catalysis are interested in whether conducting reactions at nanoparticle surfaces would compare favorably with those at macroscopic surfaces. However, the high surface area of nanoparticles sometimes makes them thermodynamically unstable relative to bulk material. Passivating nanoparticle surfaces with ligands can help, but even then there is an inevitable trade-off between nanoparticle stability and surface access.
Graduate student Youngjong Kang and Professor T. Andrew Taton have recently shown that
access of molecules to a nanoparticle surface can be regulated by a self-assembled, block-copolymer shell whose permeability varies with solvent
conditions (
However, in solvents that normally dissolve polystyrene, the glassy polymer block swells with solvent, and the cloak becomes suddenly (though reversibly) permeable. In this way, molecules that have been excluded from the nanoparticle core, like the cyanide etchant shown in the figure below, can suddenly access the particle surface. As THF swells the hydrophobic polymer, it carries dissolved cyanide with it and, in this case, dissolves away the encapsulated particle. In general, crosslinked polymer shells could be useful not only to protect sensitive particles, but also to control access of catalytic nanoparticles to substrates, or of sensing particles to analytes.
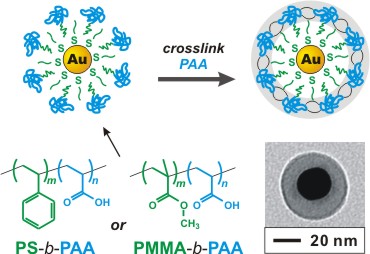
Figure 1. Encapsulation of Au nanoparticles within crosslinked, block-copolymer shells. The polyacrylate block is crosslinked with a diamino-oligo(ethylene oxide), which imparts broad solubility to the particles. In principle, any glassy block can be used in the core, and the specific polymer used dictates which solvents permeate the shell and which do not.
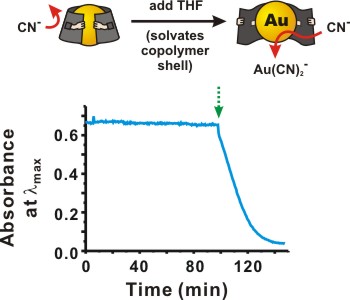
Figure 2. Solvent-triggered etching of encapsulated nanoparticles. Even in the presence of an overwhelming excess of cyanide ion, PS-b-PAA-coated particles remain stable (and deep red in color) in solution. However, when 20% THF is added, which solvates the PS block, the encapsulated particles are quickly etched away.
Next scheduled update: Jan 4th, 2004.
Copyright 2004 by the Regents of the University of Minnesota.For questions or comments, contact the Chemistry Webmaster or read the University's Online Privacy Statement.