
Recent Research Developments
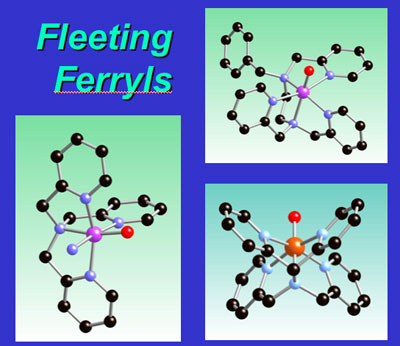
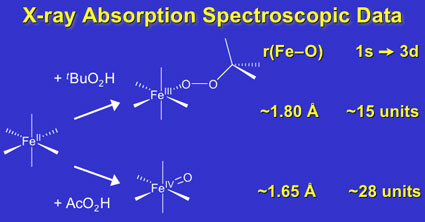
In a paper recently published in the Journal of the American Chemical Society, postdoctoral associate Jan-Uwe Rohde and co-workers in the group of Professor Lawrence Que report X-ray absorption spectroscopic studies of mononuclear low-spin alkylperoxoiron(III) and oxoiron(IV) complexes (published online as an ASAP article on December 2, 2004). These highly reactive species model intermediates proposed in the activation of dioxygen by nonheme iron enzymes. The biomimetic complexes have been generated by oxidation of synthetic iron(II) complexes of neutral tetradentate (TPA) and pentadentate (N4Py, Bn-TPEN) ligands. Notable trends observed are Fe-O bond lengths of ~1.78 Å for the Fe(III)-OOR intermediates and ~1.65 Å for the Fe(IV)=O intermediates, reflecting different strengths in the Fe-O p interactions. The intensities of the 1s-to-3d transitions also increase from 4 units for the nearly octahedral Fe(II) precursor to 9 - 15 units for the Fe(III)-OOR intermediates to 25 - 29 units for the Fe(IV)=O species, reflecting increasing distortion from centrosymmetry. This is the first systematic structural study of a series of iron intermediates related to mononuclear nonheme iron enzymes.
Next scheduled update: Dec 22nd, 2004.
Copyright 2004 by the Regents of the University of Minnesota.For questions or comments, contact the Chemistry Webmaster or read the University's Online Privacy Statement.