
 |
|
Recent Research Developments |
|
| Index of Recent Research News |
| February 4th, 2004 |
|

While sponges are considered to be the most primitive
of multicellular animals, they continue to be a rich source of complex,
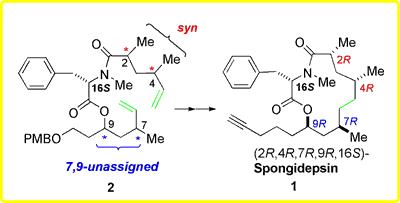
biologically active organic molecules. Spongidepsin (1) is a
remarkable recent example isolated from a Spongia sp sponge
collected off of the Vanuatu Islands, Australia, by Riccio and co-workers
in Italy.2 Its cytotoxic and antiproliferative activities
against cancer cell lines are accompanied by an unprecedented structure
that combines amino acid and unprecedented ketide motifs within a 13-membered
macrocycle. The ketide domain is comprised of a 9-hydroxy-2,4,7-trimethyltetradeca-14-ynoic
acid, while the amino acid has been established as (S)-N-methylphenylalanine.
The dimethyl substitution at C2 and C4 was originally determined to
be syn, but the absolute configuration
was not established. Neither the relative, nor the absolute stereochemistry
of the two remaining stereogenic centers at C7 and C9 were
originally assigned. Hence, the actual structure of 1 could
have been one of eight possible stereoisomers |
|
|
Collected in Australia, and isolated in Italy, the complete structure of 1 was fully established in Minnesota. For this, a stereodivergent total synthesis strategy that features macrocycle formation via ring closing metathesis (RCM) as the key step was employed. The stereochemical determination strategy relied upon the preparation of all eight possible diastereoisomers of the macrolide-containing portion of spongidepsin incorporating (S)-N-methylphenylalanine. These include both (2S,4S)- and (2R,4R)-enantiomers of the syn-2,4-dimethyl moiety conjoined with the four diastereomeric combinations of (R,S)-isomers at C7 and C9. Comparison of the spectral data of each of the diastereomeric probes with those of natural spongidepsin indicated that a specific isomer was to be selectively advanced to the total synthesis of 1. The 13-membered macrolides were prepared by RCM of dienes 2 and subsequent alkene hydrogenation. The RCM substrates 2, in turn, were derived from syn-2,4-dimethyl and C7,C9-containing fragments representing C1-C5 (4) and C6-C11 (3) of 1, respectively. The two enantiomers of syn-2,4-dimethyl carboxylic acid 4 are derivable from acetate-alcohol 6, which was provided by Jianyan Xu, via alternative manipulations of the terminal functional groups. Each of the four stereoisomers of 3 originated from the known L-malate-derived epoxide 5 bearing a C9 stereogenic center. |

|
|
1HNMR spectrum of synthetic (2R,4R,7R,9R,16S)-1. Synthetic (2R,4R,7R,9R,16S)-1 prepared in this fashion matched natural spongidepsin by 1H and 13C NMR spectroscopy, HRMS spectrometry and specific rotation. This work highlighted the convergence of synthetic design, methodology, and spectroscopic analyses to fully define the structure and provide an alternative source of the antiproliferative natural product spongidepsin. It also reflects the involvement of Minnesota chemists in successful global scientific alliances. 1) J. Chen and C. J. Forsyth Angew. Chem. Int. Ed. Engl. 2004, in press. 2) A. Grassia, I. Bruno, C. Debitus, S. Marzocco, A. Pinto, L. Gomez-Paloma, R. Riccio, Tetrahedron, 2001, 57, 6257. We thank Prof. Riccio and co-workers for providing comparative spectroscopic data. |
| * This page is updated every two weeks. Next scheduled update: Feb. 18, 2004. |
|
Copyright 2004 by the Regents of the University of Minnesota.For questions or comments, contact the Chemistry Webmaster or read the University's Online Privacy Statement. |