
Recent Research Developments
-
Lodge Research Group
In recent years it has become apparent that amphiphilic molecules such as lipids, surfactants and block copolymers can all form three archetypical micellar aggregates in aqueous solution, namely spheres, cylinders, and vesicles. The main difference among the three structures is the interfacial curvature, which can be controlled by the architecture of the amphiphile (i.e., a longer polar headgroup means greater curvature) or by the interfacial tension (i.e., a more hydrophobic core block leads to a flatter interface). Surprisingly, perhaps, this curvature sequence has not been reported in standard hydrocarbon block copolymer micelles, such as poly(styrene-b-isoprene) in organic solvents, although such systems have been studied for almost 50 years. We have recently shown, however, that this sequence can be observed by suitable choice of block length, and by varying interfacial tension through mixtures of homologous solvents. In the example shown below, various dialkyl phthalates (butyl, ethyl, and methyl) were mixed in various proportions. With decreasing alkyl length, the solvent becomes more "isoprene-phobic", and the interfacial tension goes up, leading to the sequence spheres/cylinders/vesicles.

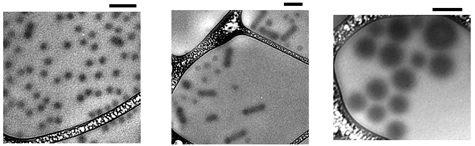
Cryo-transmission electron micrographs of micelles formed by a polystyrene-polyisoprene block copolymer, with block molar masses of 13,000 and 71,000 g/mol, respectively,dissolved at 1% concentration in various dialkyl phthalate solvents. Scale bar is 200 nm. Cartoons indicate the chain packing within the various structures.
Next scheduled update: Sept 29th, 2004.
Copyright 2004 by the Regents of the University of Minnesota.For questions or comments, contact the Chemistry Webmaster or read the University's Online Privacy Statement.