
Recent Research Developments
-
Stable Dioxetane Precursors as Selective Trap-and-Trigger Chemiluminescent Probes for Singlet Oxygen
Singlet oxygen (1O2), the lowest excited state of molecular oxygen, is a short-lived reactive oxygen species (ROS) that is formed in both biological and aqueous environmental systems via both ?light? (photosensitized) and ?dark? processes. Singlet oxygen is a known cytotoxin, believed to be involved in DNA damage, lipid peroxidation, and the bactericidal response of certain antibodies. In sunlit surface waters, 1O2 reacts with several types of organic micropollutants, such as some pharmaceuticals and pesticides. An ability to detect and quantify the production of 1O2 in such systems allows for a better understanding of the mechanisms of oxidative damage in the body and estimations of the environmental fates of pollutants that are degraded by reaction with 1O2. Due to the transient nature of 1O2 (it has a lifetime of ~ 4 μs in water) and its very low concentrations (10-15 – 10-12 M steady-state concentrations are typical in sunlit surface waters), a highly sensitive detection method is needed. Since 1O2 is often formed simultaneously with other ROS, such as superoxide radical anion, hydroxyl radical, and peroxides, it is also imperative that the detection method be selective for 1O2 relative to other ROS. Graduate students Laura MacManus-Spencer, Douglas Latch, and Kim Kroncke, along with Professor Kristopher McNeill, have developed a sensitive and selective trap-and-trigger detection method based on the trapping of 1O2 to form a stable dioxetane intermediate that, when chemically triggered, emits a luminescent signal that is proportional to the amount of 1O2 trapped.
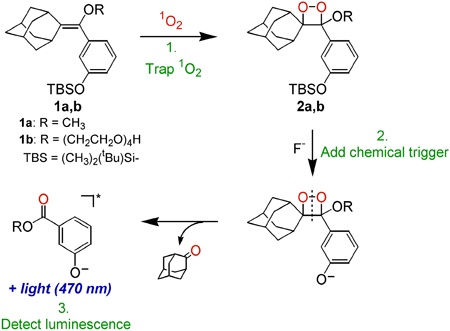
Figure 1. In this chemiluminescent detection scheme, 1O2 is trapped with probe 1 to form a thermally stable dioxetane (2), which emits visible light (470 nm) upon addition of a chemical trigger (F-).
The selectivity of this detection method for 1O2 is afforded by the formation of a stable dioxetane intermediate upon the addition of 1O2 to a spiroadamantyl-substituted vinyl ether probe molecule, as a dioxetane is a characteristic product of the reaction of 1O2 with an electron-rich alkene and is unlikely to be formed by other ROS. The selectivity of probe 1a for 1O2 with respect to superoxide radical anion (O2-?) and hydrogen peroxide (H2O2), compared to another chemiluminescent probe for ROS (MCLA = methyl Cypridina luciferin analog), is illustrated in Figure 2a.The sensitivity of the detection method lies in the chemiluminescent detection of the aryloxy emitter formed in the fluoride ion-triggered decomposition of the dioxetane. The trap-and-trigger nature of this detection scheme represents a facile method to measure photochemically generated 1O2, as it allows for low background chemiluminescence measurements in the dark after trapping 1O2 in the light. It also facilitates kinetic measurements of 1O2 formation as a function of time.
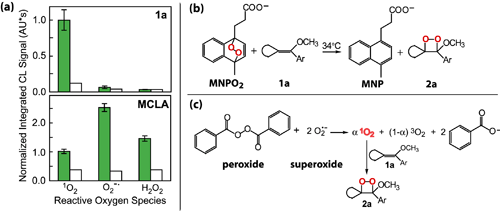
Figure 2. (a) Comparison of the chemiluminescence (CL) signals elicited from 5 μM MCLA and 10 μM 1a when exposed to three ROS: 1O2, O2-?, and H2O2. Filled columns with error bars represent the average and one standard deviation for three measurements, and the unfilled columns represent the blank signal in each system. Probe 1a was used to detect 1O2 (b) in the thermal decomposition of a naphthalene endoperoxide, MNPO2, and (c) in the reaction of benzoyl peroxide with O2-?.
The detection scheme described here has been used in the McNeill lab to quantify both photochemically and thermally generated 1O2 in aqueous and hydrophobic environments. Probes 1a and 1b were used to detect and quantify 1O2 generated photochemically using a small-molecule model photosensitizer. Using results from these experiments, it has been shown that steady-state concentrations of 1O2 as low as 5 ? 10-13 M can be measured accurately with an exposure time of ten minutes. The detection scheme has been successfully applied to natural water samples (for example, Lake Superior surface water) to measure environmentally relevant 1O2 steady-state concentrations.
The application of probe 1a to the detection of thermally generated 1O2 was tested using a water-soluble naphthalene endoperoxide, which releases 1O2 when heated (Figure 2b). Subsequently, the selectivity of the detection scheme for 1O2 has been exploited to investigate ?dark? 1O2 generating reactions involving other ROS. Singlet oxygen was detected and quantified in the reaction of O2-? with benzoyl peroxide in organic solvent (Figure 2c), a model system for ?dark? biological reactions involving ROS in hydrophobic environments, such as lipid bilayers. The formation of 1O2 in reactions involving other ROS has important implications for understanding, for instance, the mechanisms of cytotoxicity in organisms under oxidative stress.
The trap-and-trigger chemiluminescent 1O2 detection scheme was described in a recent publication (L. A. MacManus-Spencer, et al.,Analytical Chemistry, 2005, http://dx.doi.org/10.1021/ac048293s ) and will be highlighted in the March issue of ?Chemistry World? (http://www.rsc.org/chemistryworld/news/free/cw005001cs.htm).
Next scheduled update: Feb. 16th, 2005
Copyright 2004 by the Regents of the University of Minnesota.For questions or comments, contact the Chemistry Webmaster or read the University's Online Privacy Statement.