
Recent Research Developments
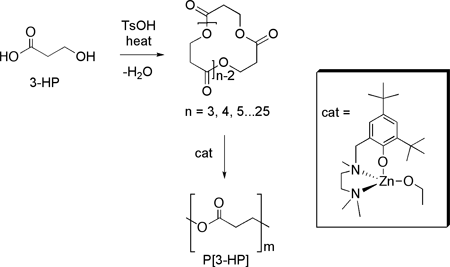
Dr. Donghui Zhang, a postdoctoral research associate working in the laboratories of Professors Marc Hillmyer and William Tolman, has developed a new procedure (Scheme 1) for preparing high molecular weight poly[3-hydroxypropionic acid] (P[3-HP]), a biorenewable and biodegradable plastic with attractive mechanical properties. In a paper recently published in Macromolecules [1] Donghui showed that 3-HP macrocyclic esters derived directly from 3-hydroxypropionic acid, a biorenewable resource resulting from the fermentation of sugars, could be readily polymerized by a Zn-based catalyst recently developed in the Hillmyer/Tolman laboratories [J. Am. Chem. Soc. 2003, 125, 11350]. Previously reported methodologies for preparing P[3-HP] are difficult to control or require the use of toxic monomers. This new procedure circumvents both of these problems and can be readily performed on a large scale.
Specifically, a 3-HP macrocylic ester mixture was obtained from a simple one-pot condensation reaction of a commercially available aqueous solution 3-HP. The 3-HP trimer (Scheme 1, n = 3) was isolated from the mixture and polymerization of this 12-membered ring occurred rapidly at room temperature to give high molecular weight P[3-HP] samples (up to 67 kg⋅mol-1). Moreover, it was shown that 3-HP cyclic esters with larger ring sizes (n = 4 or 5) also could be polymerized. These results demonstrate the practicality of this new synthetic route to an environmentally friendly plastic.
[1] Zhang, D.; Hillmyer, M. A.; Tolman, W. B. “A New Synthetic Route to Poly[3-hydroxypropionic acid] (P[3-HP]): Ring-Opening Polymerization of 3-HP Macrocyclic Esters” Macromolecules 2004, 37, 8198
Next scheduled update: April 13th, 2005.
Copyright 2005 by the Regents of the University of Minnesota.For questions or comments, contact the Chemistry Webmaster or read the University's Online Privacy Statement.