
Recent Research Developments
Selective chemical modification of biological molecules has become a powerful tool in the field of biotechnology. It is vital that specific modifications are selective for a desired functional group within a biomolecule, while at the same time unreactive with other functional groups. By using the Stuadinger reaction, which covalently ligates an azide to a phosphine, we are able to site specifically label peptides.
To incorporate the orthogonal azide functional group into peptides, the Distefano group utilizes the protein farnesylttransferase enzyme. This enzyme catalyzes the transfer of isoprenoid moieties to specific proteins possessing a C-terminal tetrapeptide, CaaX. A geranylazide diphophate compound (1 in Figure 1) was synthesized and was found to be a substrate for PFTase. The azide moiety, via the isoprenoid group, was attached to a cysteine residue within a peptide substrate containing the CaaX box motif. (Figure 1) Next, the ability of the azide-functionalized peptide to undergo a Staudinger ligation was studied. To accomplish this, the azylated peptide (2) was treated with the phosphine reagant (3), yielding product 4. Suprisingly, the product contained an O-alkyl imidate-linkage between the phophine and azide, unlike the conventional amide-linked product observed by others.1 Current studies are aimed at further characterizing this product and determining the mechanism of its formation.
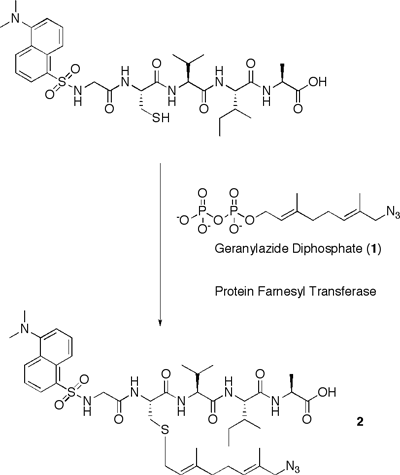

Incorporation of an azide into a peptide and subsequent Staudinger ligation reaction
These results demonstrate the ability to enzymatically incorporate prenyl azide groups into a peptide substrate using PFTase, and subsequently modify the peptide using a Staudinger ligation reaction. Therefore, it should be possible to site-specifically modify any protein with a CaaX tetrapeptide engineered onto its C-terminus. This strategy may be useful for several applications in protein chemistry, including biophysical probe incorporation and surface attachement.
1) Saxon, E.; Berozzi, C. R. Science 2000, 287, 2007-2010
Next scheduled update: Oct 26th, 2005.
Copyright 2005 by the Regents of the University of Minnesota.For questions or comments, contact the Chemistry Webmaster or read the University's Online Privacy Statement.