
Recent Research Developments
Crosslinked polystyrene (PS) is commonly synthesized in one step by copolymerizing styrene and a branching monomer (such as divinylbenzene). Making PS that can be crosslinked after it has been polymerized÷separating the polymerization and crosslinking steps÷requires that latent, crosslinkable functional groups be incorporated into the polymer. Graduate student Ying Chen, undergraduates Anita Tavakley and Tate Mathiason, and professor T. Andrew Taton recently reported that this can be accomplished, post-polymerization, by exposing PS-containing polymers to Lewis-acid-free Friedel-Crafts reagents (J. Polym. Sci. Part A, Polym. Chem. 2006, 44, 2604). For example, the benzene rings of PS-containing homo- and co-polymers can be stoichiometrically converted to photocrosslinkable benzophenone groups using benzoyl triflate (BzOTf). Importantly, this reagent did not damage Lewis-acid sensitive components of the polymer, including poly(ethylene oxide)- and polyacrylate blocks, that were destroyed under typical Lewis-acid catalyzed Friedel-Crafts conditions.
Chen and coworkers then demonstrated that the resulting poly(vinylbenzophenone)-containing polymers could be crosslinked under UV light exposure to form permanent networks that were not dissolved by organic solvents. This reagentless crosslinking method was used to form permanent micelles, vesicles, and gels that retained the shapes and morphologies of the pre-crosslinked materials. In principle, because styrenic polymers are so common, this route could provides a quick and easy avenue to functional polystyrenes without incorporating complex monomers into the original styrene polymerization.
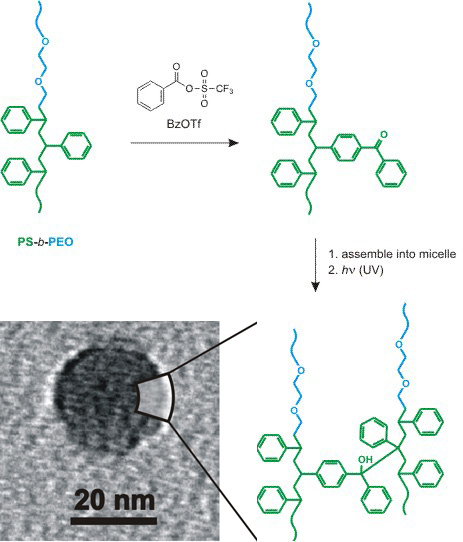
Figure 1. Poly(styrene-block-ethylene oxide) can be converted to a photocrosslinkable, poly(vinylbenzophenone)-containing amphiphile by Friedel-Crafts acylation with BzOTf. Permanently crosslinked micelles can then be formed from this amphiphile by assembly followed by exposure to UV light.
Next scheduled update: April 26th, 2006.
Copyright 2006 by the Regents of the University of Minnesota.For questions or comments, contact the Chemistry Webmaster or read the University's Online Privacy Statement.