
Recent Research Developments
- J. Pu, S. Ma, M. Garcia-Viloca, J.
Gao, and D. G. Truhlar
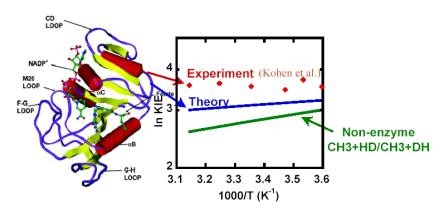
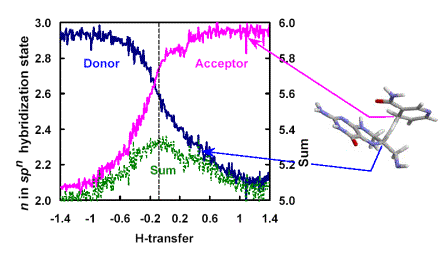
As a target for anti-cancer and anti-bacterial drugs, dihydrofolate reductase (DHFR) plays a critical role in maintaining the intracellular concentration of tetrahydrofolate (THF), an important reductive cofactor in biosynthesis of DNA building blocks and several amino acids. The H/D kinetic isotope effects (KIEs) for the E. coli DHFR-catalyzed hydride transfer display an unusually small dependence on temperature, which is contrary to experience with small-molecule chemistry or simple tunneling models. Free energy simulations based on a combined QM/MM potential and KIE calculations employing ensemble-averaged variational transition state theory with multidimensional tunneling (EA-VTST/MT) indicated that the small temperature dependence results not from some intrinsically temperature-independent mechanism but rather from a near cancellation of competing temperature-dependent effects. The theoretical study also revealed an interesting non-perfect synchronization of reaction center rehybridizations during the hydride transfer, where the rehybridization of the hydride-acceptor carbon is significantly advanced compared to that of the hydride-donor carbon, resulting in a compressed transition state.
Next scheduled update: Jan 18th, 2006.
Copyright 2006 by the Regents of the University of Minnesota.For questions or comments, contact the Chemistry Webmaster or read the University's Online Privacy Statement.