
Recent Research Developments
Large-scale analysis in biotechnology often involves the use of solutions of small volumes, e.g., nanoliter droplets used in protein microarray fabrication. An implicit assumption is that molecules in a nanodroplet behave the same as in bulk solution. A recent study by graduate student Yang Deng and Chemistry Professor Xiaoyang Zhu, in collaboration with Taryn Kienlen and Dr. Athena Guo of MicroSurfaces, Inc., showed that this assumption was invalid because of the much increased surface area-to-volume ratio (A/V) for a nanoliter size droplet (Y. Deng, X.-Y. Zhu, T. Kienlen, A. Guo J. Am. Chem. Soc. 128 (2006) 2768-2769). The large A/V value leads to the accumulation of protein molecules at the air-water interface and a depletion of protein concentration inside the droplet. Thus, when considering chemical kinetics in a nanodroplet of protein solutions, we must now include the dominant role played by the air-water interface. A common manifestation of this new mechanism is the mysterious occurrence of rings in most protein microarrays, a result of the much enhanced reaction probability at the perimeter of a nanodroplet on the surface. The occurrence of these non-uniform spots prohibits quantitative analysis and is detrimental to protein microarray applications. These authors demonstrates that the ring structure in protein microarray can be transformed into uniform spot profiles by the addition of a small amount of detergent molecules that displace proteins from the air-water interface, Figure 1. The fundamental difference between a nanoliter droplet and bulk solution must be kept in mind when we address various aspects of chemical process involving protein molecules in a nanoliter droplet.
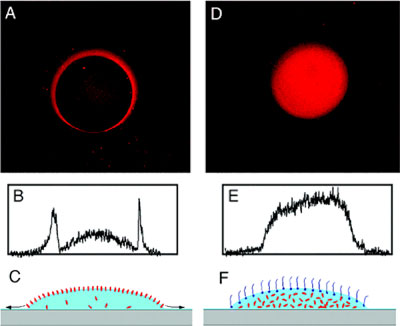
Figure 1 (A and D): Fluorescence microscope images of antibody (rabbit polyclonal anti-vanilloid receptor 1) spots immobilized on an epoxy-functionalized glass slide. In (A), a diluted antibody solution (1:500) was used directly, while in (D), a small amount detergent (0.006% Triton X-100) was added to the diluted antibody solution. Panels (B) and (E) are cross-sectional profiles of images (A) and (D), respectively. Panels (C) and (F) are schematic illustrations of nanoliter droplets (light blue) on a solid surface (gray) with protein molecules in red and detergent molecules in dark blue. Diameter of each spot is ~0.1 mm.
Next scheduled update: July 5th, 2006.
Copyright 2006 by the Regents of the University of Minnesota.For questions or comments, contact the Chemistry Webmaster or read the University's Online Privacy Statement.