
Recent Research Developments
Approximately 300 genera of Angiosperms produce rubbers of high molecular weight. Among them, Hevea brasiliensis (Brazilian rubber tree) is the highest producer, making it the most common source for commercial cultivation. The latex of Hevea contains rubber particles known to initiate and maintain the synthesis of cis-1,4-polyisoprene. Despite years of intense research, the identity of the enzyme(s) responsible for rubber biosynthesis in plants such as Hevea is still unknown. Such information is critical for the development of enhanced rubber producing plants and/or organisms for commercial natural rubber cultivation.
The Distefano group has developed benzophenone-based isoprenoid pyrophosphate mimics that have shown to be useful in the photoaffinity labeling of the prenyltransferase enzymes protein farnesyltransferase and protein geranylgeranyltransferase type I. In collaboration with Dr. Colleen McMahan at the USDA's division of Crop Improvement & Utilization Research and Dr. David Shintani, Dept. of Biochemistry, University of Nevada, Reno, researchers in the Distefano lab have been exploring the utility of such compounds for the photoaffinity labeling of potential contributors to rubber biosynthesis in isolated rubber particles from Hevea latex. Recent results demonstrate that the 32P-labeled analog 1 specifically photolabels distinct bands within a complex mixture of rubber particle proteins (Figure 1).
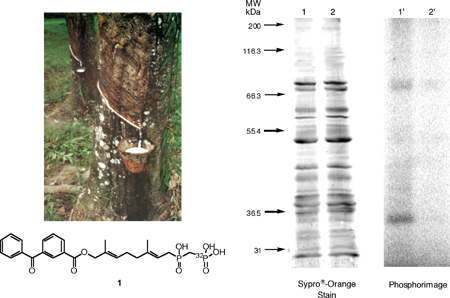
Figure 1. Upper Left: A freshly tapped Hevea brasiliensis tree collecting latex milk. Lower Left: 32P labeled benzophenone-based isoprenoid pyrophosphate mimic. Right: Analysis of photolabeling of Hevea washed rubber particles (WRPs) with 1 by SDS-PAGE. Lanes 1 and 1' contain samples of WRPs and 1 irradiated at 350 nm. Lanes 2 and 2' contain samples of W RPs and 1 irradiated in the presence of farnesyl pyrophosphate. Lanes 1 and 2 show proteins identified with Sypro©-Orange staining. Lanes 1'and 2' show radiolabeled proteins identified with phosphorimaging
Subsequent isolation and mass spectral analysis of these bands identified the enzyme Rubber Elongation Factor (REF), which is known to be a major protein constituent in Hevea latex. Other peptide fragments that could not be attributed to REF were also seen, and MS/MS analysis is currently underway in order to identify their origin. This data should facilitate the cloning of the genes responsible for rubber biosynthesis and potentially eliminate our dependence on synthetic rubber.
Next scheduled update: April 12th, 2006.
Copyright 2006 by the Regents of the University of Minnesota.For questions or comments, contact the Chemistry Webmaster or read the University's Online Privacy Statement.