Main navigation | Main content
“Serendipity—discovering by accident something good or useful that was not being searched for.” It is not surprising that serendipity occurs a lot in the scientific community. But it takes curiosity, knowledge, wisdom, and tenacity to prevent that serendipitous discovery from being overlooked or dismissed as a mistake.
Professor Thomas Hoye and members of his research team have uncovered the heretofore-overlooked scope of an important chemical reaction, which they have named the hexadehydro-Diels-Alder (HDDA) reaction. Discovering new modes of chemical reactivity is rare and is so unprecedented that the University of Minnesota is seeking a patent for it.
Hoye’s reaction builds on the work of Otto Paul Hermann Diels and Kurt Alder, who first documented what is called the Diels-Alder reaction in 1928. They received a Nobel Prize for their work in 1950. Every chemist knows about the Diels-Alder reaction. It is presented in every chemistry textbook and taught to every organic chemistry student.
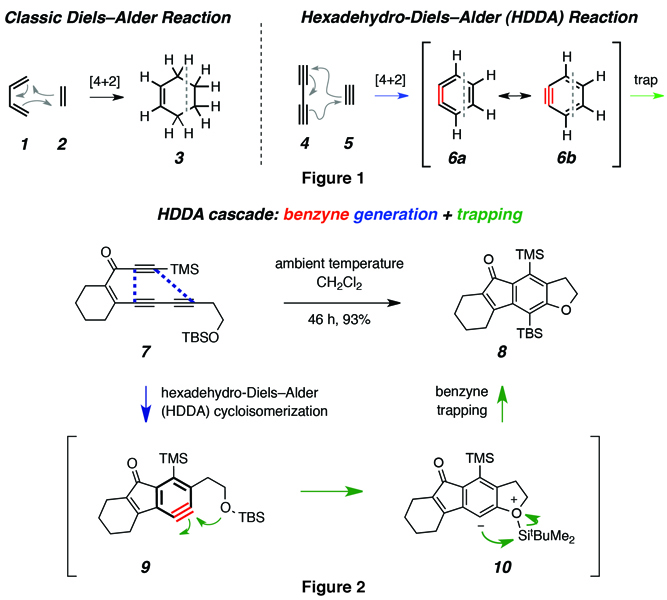
The new HDDA reaction is a variant of that classic Diels-Alder reaction (Figure 1). In the typical Diels-Alder reaction, six-membered rings (cyclohexenes, 3) are formed by the reaction of two precursors—a diene (1) and a dienophile (2). The hexadehydro-Diels-Alder reaction involves, instead, the cycloaddition of a conjugated diyne (4) with another alkyne, the “diynophile” (5 ). But this seemingly subtle difference has a huge, beneficial ramification. The product is, itself, a highly reactive species called a benzyne (6a/6b).
Benzynes are one of the most widely studied and useful of all “reactive intermediates” encountered in organic chemistry. However, because of the preparative requirements for generating benzynes, they can be challenging to make. In addition, because of the other external reagents, which are other additives needed to bring about chemical reactions, and byproducts such as metals and bases that accompany the typical methods for preparing benzynes, certain types of benzyne trapping reactions are not feasible. In contrast, the two-stage HDDA cascade reaction (Figure 2) merely involves heating the appropriate triyne precursor (e.g., 7) in the presence of the benzyne trapping agent; no other reagents need to be present. Thus, the HDDA process represents a highly complementary and orthogonal method for constructing the high-energy benzynes (e.g., 9, enroute to 8 via 10).
Benzynes are highly valued because of their remarkable efficiency and ability to react with other chemical substances. They are used to build structurally complex benzenoid products (cf. 8), which are stable, versatile, synthetic organic compounds used in the development of pharmaceuticals, including many marketed drugs (i.e., pharmaceutical agents), agrochemicals, dyes, and polymers.
Post-doctorate researcher Beeraiah Baire, Ph.D., unexpectedly encountered the first example of the HDDA reaction during the course of an unrelated study in July 2011.
“We could have easily talked ourselves out of this,” said Hoye. “We sensed that we were on to something, but we initially didn’t know what it was. Something instinctively told us that this was an important observation and discovery.”
Since last summer, Hoye, Baire, and graduate students Dawen Niu, Patrick Willoughby, and Brian Woods have been using the HDDA reaction to create benzynes and have been studying the resulting new reactivity patterns. Hoye hopes that this ongoing research will lead to new discoveries for the use of benzynes. One example is the development of compound libraries useful to support drug discovery efforts.
Their work on the hexadehydro-Diels-Alder reaction was published in the October 11 edition of Nature (doi:10.1038/nature11518).
Hoye is a top organic chemist and professor. He joined the University of Minnesota chemistry faculty in 1976 and has received accolades for his research and his teaching, including being named the Merck Professor of Chemistry in 2002, being recognized as a Distinguished Graduate Teaching Professor in 1999, and receiving the Horace T. Morse University of Minnesota Alumni Association Award for Outstanding Contributions to Undergraduate Education in 2007. More than 130 undergraduates students have done independent research studies in his laboratories. Twelve masters and 74 doctoral graduate students have carried out their thesis research under his guidance, and dozens of postdoctoral and visiting scientists have studied in his group.
The primary emphasis of Hoye’s research group is the development of new strategies for natural product total synthesis.