Main navigation | Main content
The latest discovery by researchers working with Professor Gianluigi Veglia may aid in designing innovative therapeutic approaches to heart failure. This research, "Allosteric regulation of SERCA by phosphorylation-mediated conformational shift of phospholamban," was published in the Proceeding of the National Academy of Science and highlighted in Nature Chemical Biology.
Authors include Veglia from the departments of Chemistry and Biochemistry, Molecular Biology & Biophysics (BMBB); Martin Gustavsson, Raffaello Verardi, Daniel G. Mullen, ant T. Gopinatha from BMBB; Kaustubh R. Mote from the Department of Chemistry; and Nathaniel J. Traaseth, BMBB graduate student who is now a postdoctorate and New York University.
According to the abstract for the significance for this research is: "The sarcoplasmic reticulum Ca2+-ATPase (SERCA)/phospholamban complex regulates cardiac muscle contractility by controlling Ca2+ transport from the cytosol to the lumen of the sarcoplasmic reticulum. By mapping the interactions between these two membrane proteins, we found that SERCA function depends on the equilibria between transient conformational states of phospholamban. Phosphorylation of phospholamban shifts the equilibria, enhancing SERCA function. This mechanism explains why tuning phospholamban’s structural dynamics can modulate SERCA function and may aid in designing innovative therapeutic approaches to heart failure."
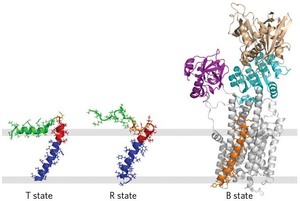
According to the abstract: "The membrane protein complex between the sarcoplasmic reticulum Ca2+-ATPase (SERCA) and phospholamban (PLN) controls Ca2+ transport in cardiomyocytes, thereby modulating cardiac contractility. β-Adrenergic-stimulated phosphorylation of PLN at Ser-16 enhances SERCA activity via an unknown mechanism. Using solid-state nuclear magnetic resonance spectroscopy, we mapped the physical interactions between SERCA and both unphosphorylated and phosphorylated PLN in membrane bilayers. We found that the allosteric regulation of SERCA depends on the conformational equilibrium of PLN, whose cytoplasmic regulatory domain interconverts between three different states: a ground T state (helical and membrane associated), an excited R state (unfolded and membrane detached), and a B state (extended and enzyme-bound), which is noninhibitory. Phosphorylation at Ser-16 of PLN shifts the populations toward the B state, increasing SERCA activity. We conclude that PLN’s conformational equilibrium is central to maintain SERCA’s apparent Ca2+ affinity within a physiological window. This model represents a paradigm shift in our understanding of SERCA regulation by posttranslational phosphorylation and suggests strategies for designing innovative therapeutic approaches to enhance cardiac muscle contractility."