Main navigation | Main content
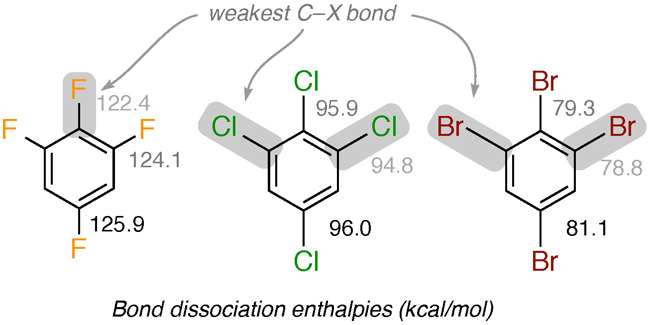
With the increased usage of fluorinated aromatics in industry and medicine, interest in fluorinated aromatics as environmental pollutants has increased. While it is well known that carbon-fluorine bonds are stronger than other carbon-halogen bonds and that this affects their susceptibility to degradation in the environment, it has not been generally recognized that fluorinated aromatics also manifest unique trends in their thermochemical properties that make them different from analogous chlorinated and brominated aromatics, particularly from the standpoint of environmental chemistry.
In collaboration with Professor Christopher Cramer and Professor Kristopher McNeill (now of ETH Zurich), theoretical calculations by graduate student Daniel Sadowsky, recently published in Environmental Science & Technology, have demonstrated that carbon-fluorine bond strengths in aromatic systems exhibit significantly greater variation than do carbon-chlorine and carbon-bromine bonds. Moreover, the trends that are observed in the bond strengths of fluorinated aromatics often run counter to the trends observed in chlorinated and brominated aromatics. Similarly divergent trends are predicted for related thermochemical properties, and can largely be attributed to the smaller atomic size of fluorine and the increased polarity of the carbon-fluorine bond relative to the heavier halogens.
The paper can be viewed at dx.doi.org/10.1021/es404033y.