Main navigation | Main content
Atmospheric chemists have long recognized the crucial role the role that sulfuric acid plays in initiating the formation of atmospheric aerosol. Its immediate precursor in the atmosphere is SO3 and, for this reason, the H2O + SO3 interaction has long been of interest. More recently, the high concentrations of organic matter found in atmospheric aerosol, together with the observation of high measured concentrations of atmospheric carboxylic acids, have set the stage for additional interest in SO3 − carboxylic acid interactions.
In a recent paper, Science 3, July (2015), Graduate Student Rebecca Mackenzie, Postdoctoral Associate Christopher Dewberry, Ph.D., and Professor Kenneth Leopold have reported the production and microwave spectroscopic characterization of the anhydride formed from SO3 and formic acid.
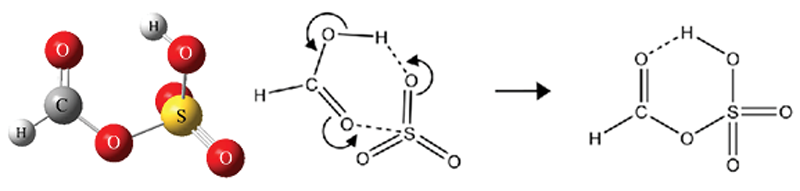
Formic sulfuric anhydride (FSA) has the structure shown (left below) and was observed to form readily during the first few tens of microseconds of a supersonic expansion. High level ab initio calculations further indicate that the production from SO3 and HCOOH is exothermic and that the zero point corrected barrier is only 0.2 kcal/mol. A π2 + π2 + σ2 cycloaddtion reaction within the SO3−HCOOH van der Waals complex (right below) is a plausible mechanism for FSA formation and additional calculations on pinic and benzoic acid indicate that the reaction should occur for other carboxylic acids as well. While the atmospheric significance of FSA remains to be seen, one possible role might involve facile formation followed by hydrolysis in small water containing clusters and/or droplets as a mechanism to deposit carboxylic acids into atmospheric aerosol. The literature on mixed carboxylic acid – sulfuric acid anhydrides is sparse, and at the very least, this work provides definitive spectroscopic evidence for a rather unusual and unexpected chemical species.
R.B. Mackenzie, C.T. Dewberry, and K.R. Leopold, Science, Vol. 349, No. 6243, 3 July (2015), pp. 58-61. DOI:10.1126/science.aaa9704