Main navigation | Main content
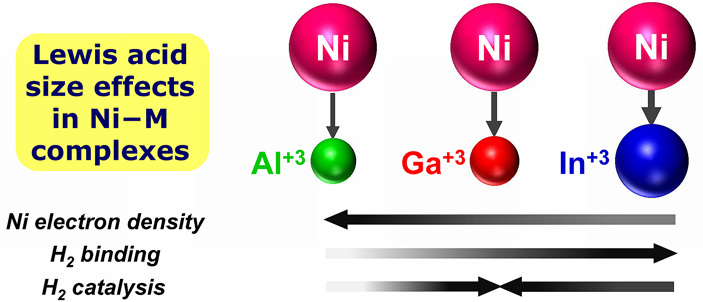
In a new study, chemists at the University of Minnesota reveal how catalytic activity can be dramatically tuned by using the appropriately sized ancilliary ion. Specifically, the nickel bimetallic complexes, where an electron-rich nickel(0) center is appended by a Group 13 metal ion, show significantly different properties and reactivities that ultimately depend on the size of the ancillary ion. The larger ions, Ga(III) and In(III), effectively promote Ni-based activity, whereas the smaller Al(III) did not. Single nickel centers are rare as active sites in hydrogenation, even though Raney nickel, a Ni-Al alloy, is used industrially to hydrogenate arenes.
The study was conducted by graduate student Ryan Cammarota under the supervision of Professor Connie Lu, and was recently published in the Journal of the American Chemical Society.
The work was supported in part by the U.S. Department of Energy, Office of Basic Energy Sciences, Division of Chemical Sciences under award DE-SC0012702 (Inorganometallic Catalyst Design Center). Ryan Cammarota was supported by a Departmental Excellence Fellowship (Phillips 66).