Main navigation | Main content
11/24/2015
Recent research from the research group of Professor
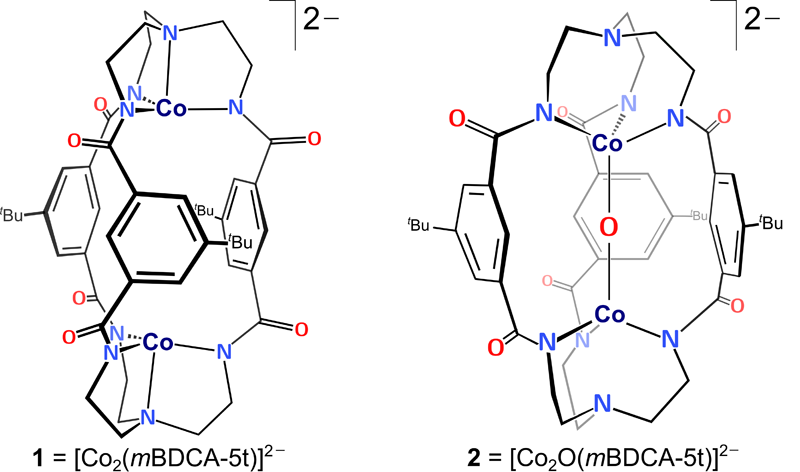
Linear metal-oxo-metal complexes are rare in coordination chemistry. However, for the first time, a unique linear Co-O-Co core encapsulated inside a cryptand ligand has been isolated and characterized.
This research represents collaborative work among the groups of Professor Laura Gagliardi from the University of Minnesota, Professor Christopher C. Cummins from the Massachusetts Institute of Technology, and Professor Daniel G. Nocera from Harvard University.
The electronic structure of the Co-O-Co complex was elucidated by Post-doctoral Associate Konstantinos D. Vogiatzis, Ph.D., in Professor Gagliardi’s group. The computational analysis was based on multiconfigurational theory and showed that the O-atom transfer does not oxidize the Co(II) centers. The bridging oxygen has an oxyl radical character and an electron hole is delocalized in the carboxamide groups of the cryptand ligand. K-edge X-ray absorption spectroscopy data were consistent with a +2 oxidation state assignment for cobalt in both complexes and verified the computational result. The Co-O-Co complex acts as a two-electron oxidant towards substrates including CO and H2, in both cases efficiently regenerating the starting dicobalt complex in what represent net oxygen atom transfer (OAT) reactions. This dicobalt system also functions as a catalase upon treatment with H2O2.
The conclusions of this collaborative work ware recently published in the Journal of the American Chemical Society.
This project was supported in part by the U. S. Department of Energy, Office of Basic Energy Sciences, under SciDAC grant no. DE-SC0008666.