Main navigation | Main content
12/20/2013
Recent research from the research group of Professor
In a paper published in Inorganic Chemistry, researchers report a study of the kinetic and mechanistic details of the ring-opening polymerization of ε-caprolactone by aluminum salen complexes. The work was a joint collaboration between the groups of professors William Tolman and Christopher Cramer with experimental work done by Maria Miranda and Yvonne DePorre, and theoretical work done by Hugo Vazquez-Lima, Ph.D., Michelle Johnson, and Daniel Marell.
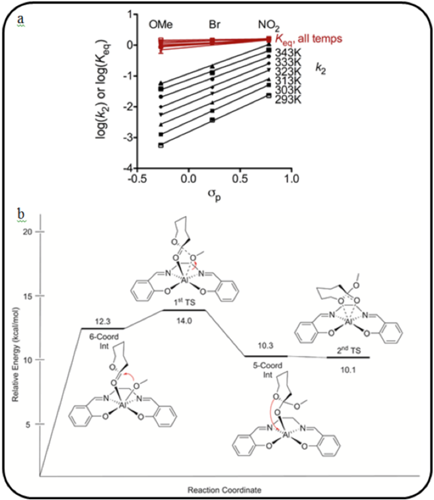
Kinetic data for a set of three Al-salen complexes, differing in their electron-withdrawing characteristics (-OMe, -Br, and -NO2), indicated the occurrence of a similar mechanism for the three complexes as well as a strong correlation between rate enhancement and increasing electron-withdrawing character of the complex. Theoretical density functional theory calculations provided a detailed analysis of the elementary steps involved in the mechanism, and showed that increasing electron-withdrawing character causes the ligation sphere around the aluminum center to tighten, thus stabilizing the transition state structure. Additionally, an alternative substrate inhibition mechanism was proposed in order to marry theoretical and experimental findings.
The paper can be found at http://dx.doi.org/10.1021/ic402255m.
"Understanding the Mechanism of Polymerization of e-Caprolactone Catalyzed by Aluminum Salen Complexes," Miranda, M. O.; DePorre, Y.; Vazquez-Lima, H.; Johnson, M. A.; Marell, D. J.; Cramer, C. J.; Tolman, W. B. Inorg. Chem. 2013, 52 (23), 13692-13701.
Combining experimental data and theoretical computations, a complete picture of the mechanistic steps involved in the ring-opening polymerization becomes clear. a) Hammett analysis indicates that electron-withdrawing substituents increase rate, and rate differences are due to differences in the alkoxide insertion step of the postulated mechanism. b) Potential energy profile of the lowest energy pathway for the ring-opening polymerization. DFT calculations indicate the rate-determining step to be alkoxide insertion and formation of the tetrahedral intermediate (1st TS), where the activation energy decreases with increasing electrophilic nature of the aluminum center.