This demonstration presents several very interesting chemical and physical pheomena.
The first reaction is an oxidation reduction reaction where copper is oxidized from the zero state to a +2 state and the nitrogen is reduced from the +5 state in nitrate to the +2 state in nitrogen monoxide. It may be pointed out that other strong acids such as hydrochloric and sulfuric do not react with copper.
The red-brown gas that appears in the round bottomed flask is caused by the same compound responsible for the red-brown haze that hangs over certain cities, nitrogen dioxide. The nitrogen monoxide reacts with atmospheric oxygen to produce nitrogen dioxide as shown in the second reaction.
There is acid-base chemistry going on. The nitrogen dioxide that is produced in the round bottomed flask is bubbled through water that has been made basic via the addition of ammonia and the condition made visible by the presence of phenolphthalein. As the nitrogen dioxide bubbles through the basic solution, the pink color disappears. This shows that the solution is no longer basic, but has become acidic. The nitrogen dioxide has been converted to nitric acid and nitrous acid as shown in the third reaction. This is the same reaction by which nitrogen oxide pollutants make acid rain.
The reaction between the copper and the nitric acid is exothermic. The contents of the round bottomed flask including the gas are at an elevated temperature.
The reaction comes to an end when the last of the copper is used up. This makes the copper the yield limiting reagent. When the reaction ceases, the bubbles of gas stop. As the contents of the round bottomed flask cool, the gas pressure decreases in the round bottomed flask. This illustrates the pressure-temperature relationship of gases. As the pressure in the roud bottomed flask decreases, the gas in the tubing is drawn back into the flask and water from the Erlenmeyer flask is also drawn through the tubing. This is a fairly slow process since the flask cools slowly.
Nitrogen dioxide is soluble in water and is continuously dissolving in the water from the large container. Since the interface between the nitrogen oxide and the water is confined to the cross sectional area of the tubing, the dissolution proceeds slowly. When the water from the large container finally is drawn all the way through the tubing and enters the round bottomed flask, the interface become much larger and the rate of dissolution increases dramatically. Within a matter of seconds nearly the entire round bottomed flask is filled with water from the large container.
The solution now in the round bottomed flask takes on the characteristic blue color of the Cu(H2O)n 2+ (n is typically 4) which illustrates some complex ion chemistry.
3Cu(s) + 2NO3 -(aq) + 8H +(aq) 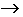 3Cu 2+(aq) + 4H2O(l) + 2NO (g)
3Cu 2+(aq) + 4H2O(l) + 2NO (g)
2NO (g) + O2 (g) 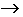 2NO2 (g)
2NO2 (g)
2NO2 (g) + H2O(l) 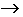 H +(aq) + NO3 -(aq) + HNO2 (aq)
H +(aq) + NO3 -(aq) + HNO2 (aq)
3HNO2 (aq) 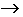 H +(aq) + NO3 -(aq) + 2NO (g) + H2O(l)
H +(aq) + NO3 -(aq) + 2NO (g) + H2O(l)

The procedure used in this demonstration is loosely based upon a pamphlet authored by Lang, Showalter and Shulfer 1. The chemistry is descibed in a book by Shakhashiri 2.
3Cu 2+(aq) + 4H2O(l) + 2NO (g)
2NO2 (g)
H +(aq) + NO3 -(aq) + HNO2 (aq)
H +(aq) + NO3 -(aq) + 2NO (g) + H2O(l)
