Separation of Food Dyes Via Column Chromatography
Equipment
10-mL syringes with luer lock tip (6) (The demonstration may be performed with only one syringe, but it is much more time consuming.), 6-well microplate, and an overhead projector
Reagents
Sep-Pak® C18 cartridge, grape flavored Kool-Aid® or grape soda, 70% isopropyl alcohol solution (minimum 20 mL), 25% isopropyl alcohol solution (minimum 10 mL), 5% isopropyl alcohol solution (minimum 10 mL), deionized water
Presentation
Preparation
- Use grape soda as it comes or make a 0.3 g Kool-Aid per 100 mL DI water solution (minimum 20 mL).
- Label the syringes as follows: "grape #1", "grape", "DI water", "70% isopropyl alcohol", "25% isopropyl alcohol #3", "5% isopropyl alcohol #2".
- Fill each of the syringes with 10 mL of the appropriate solution.
- Pretreat the column by inserting the "70% isopropyl alcohol" syringe firmly into the end of the column and pushing the solution through the column. Note: Always connect to the long end of the column.
- Do the same thing with the "DI water" syringe.
- After the demonstration, repeat steps 4 and 5 and the column may be reused indefinitely.
Demonstration
- Place the microplate on the overhead projector.
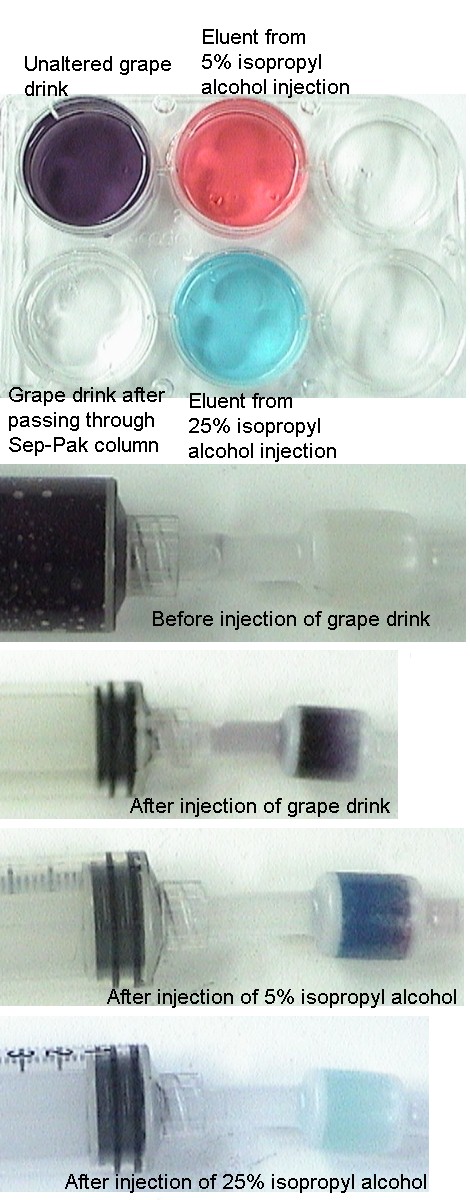
- Inject the contents of the "grape" syringe into one of the wells, note the purple color.
- Insert the "grape #1" syringe firmly into the end of the column and inject the contents into an empty well, note that the eluent is colorless. You may also note that the column now has a purple color.
- Insert the "5% isopropyl alcohol #2" syringe firmly into the end of the column and inject the contents into an empty well, note that the eluent has a red color. You may also note that the column now has a blue color.
- Insert the "25% isopropyl alcohol #3" syringe firmly into the end of the column and inject the contents into an empty well, note that the eluent has a blue color. You may also note that the column is now colorless or nearly so.
Hazards
Isopropyl alcohol is flammable and should be kept away from ignition sources, also EXPOSURE TO HIGH VAPOUR CONCENTRATIONS MAY CAUSE EYE IRRITATION. EXPOSURE TO HIGH VAPOUR CONCENTRATIONS MAY CAUSE RESPIRATORY TRACT IRRITATION, HEADACHE, DIZZINESS, NAUSEA, INCOORDINATION, DROWSINESS AND LOSS OF CONSCIOUSNESS. ALTHOUGH INGESTION IS UNLIKELY, LIQUID WOULD IRRITATE UPPER DIGESTIVE TRACT IF SWALLOWED.INGESTION OF THIS PRODUCT MAY CAUSE HEADACHE, DIZZINESS, FATIQUE AND CENTRAL NERVOUS SYSTEM DEPRESSION.
Discussion
The ingredients listed on the Kool-Aid package are: citric acid, clacium phosphate, salt, maltodextrin, modified corn starch, artificial flavo, ascorbic acid, FD&C red 40 and FD&C blue 1. The last two ingredients are the interesting components for this demonstration. This combination of red and blue dyes gives the grape drink its characteristic purple color. The structures of these dyes can be seen below.
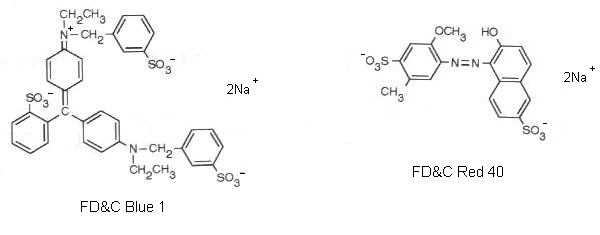
These are relatively non-polar molecules, even allowing for some charge separation from the partial ionization of the sodium ions. Red 40 is a somewhat more polar molecule than is blue 1. This can be explained by the sizes of the molecules. Each molecule will have a similar amount of charge from ionization, but since blue 1 is a larger molecule the resulting charge will be spread out over a larger molecule. The contents of the Sep-Pak column (stationary phase) are very non-polar. If the dye molecules are dissolved in a relatively polar solvent (mobile phase) such as water and the resulting solution is forced through the column, The dye molecules will preferentially associate with the stationary phase. This results in a colorless liquid (eluent) exiting the column and the column takes on the purple color from the dyes. If we decrease the polarity of the mobile phase, it should be possible to cause the dye molecules to leave the stationary phase and thus remove them from the column. A 5% isopropyl alcohol and 95% water mobile phase will remove the red 40 dye from the stationary phase and the resulting eluent will be red from the dye and the column will appear blue from blue 1 that remains on the column. A slighly less polar mobile phase consisting of 25% isopropyl alcohol and 75% water will remove the blue 1 dye from the stationary phase and the resulting eluent will be blue from the dye and the column will appear white or colorless. There may be some slight blue color if all of the dye has not been removed. The more polar molecule, red 40 is removed with the more polar mobile phase and the less polar moleculem blue 1, is removed with the less polar mobile phase.
References
- Vonderbrink, S.A., Laboratory Experiments for Advanced Placement Chemistry, Flinn Scientific: Batavia, IL, 1995, pp 149-153.
- Bidlingmeyer, B.A., Warren, F.V., J. Chem. Educ., 1984, 61, 716-720.
Request a demonstration
The University of Minnesota is an equal opportunity educator and employer.
Copyright 2000 by the Regents of the University of Minnesota.
This page was last modified 6/10/2002.
For questions or comments, contact
Joseph Franek.

