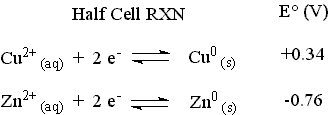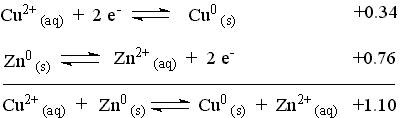Copper-Zinc Galvanic Cell
Equipment
Two 250-mL tall form beakers, strip of zinc, strip of copper, two clamps to hold metal strips, salt bridge filled with 3% agar and 1 M KCl or KNO3, voltmeter or computer interface.
Reagents
250 mL of 1.0 M CuSO4 and 250 mL of 1.0 M ZnSO4.
Presentation
- Pour one of the solutions into a beaker and the other solution into the other beaker.
- Clamp the copper strip into the beaker containing the CuSO4 solution and do the equivalent with the zinc strip.
- Connect the two beakers with the salt bridge. It is critical that the salt bridge reach beneath the surface of both solutions and that there are no air bubbles anywhere throughout the bridge.
- Connect one lead from the voltmeter to each of the metal strips. You should get a reading near 1.10 V.
Note: In order to preserve the salt bridge, after completing the demonstration, remove the salt bridge from the beakers, rinse with DI water and return it to its storage container.
Hazards
Copper Compounds can be toxic if taken internally, and dust from copper compounds can irritate mucous membranes.
Discussion
The standard reduction potentials for the two half-cells are given below.
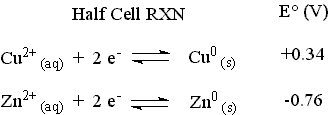
The reaction with the more positive E° proceeds as it is written (It is the cathodic reaction and the copper strip is the cathode.). The reaction with the less positive (more negative) E° will be forced to proceed in the opposite direction as it is written (It is the anodic reaction and the zinc strip is the anode.). Changing the direction of a reaction changes the sign of E° for the reaction.
Add the two reaction according to the way they will be reacting and sum the appropriate E° value to produce the overall cell reaction and the cell potential.
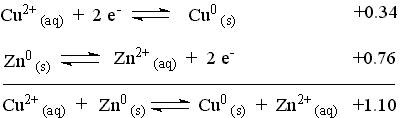
Physically, this says that the copper strip will get heavier as copper ions are reduced and deposited on its surface and the zinc strip will be getting lighter as zinc atoms are oxidized and leave the surface and enter the solution.
As can be seen in the photo, our experimental cell is 20 mV lower than the predicted 1.10 V. There are a number of factors that can account for this. The most likely factors are that the solutions are not at 1 molar concentration and that there are potential drops due to resistance to electrical conductivity in the salt bridge and the solutions.

References
- B. Z. Shakhashiri, Chemical Demonstrations, A Handbook for Teachers of Chemistry, Wisconsin, 1992,Vol. 4, p.189.
Request a demonstration
The University of Minnesota is an equal opportunity educator and employer.
Copyright 2000 by the Regents of the University of Minnesota.
This page was last modified 3/26/2000.
For questions or comments, contact
Joseph Franek.