Diels-Alder Reaction
Equipment
video camera, 13 X 100 mm testtubes (3)
Reagents
0.05 M solution of anthracene in toluene (178 mg / 20 mL), 0.05 M
solution of furan in toluene (72.7 mg / 20 mL), 0.01 M solution 4-phenyl-1,2,3-triazolin-3,5-dione
(N-PTD) in toluene (35 mg / 20 mL)
Presentation
- Add 1 mL of N-PTD solution to each of the three test tubes. The first
tube is only used to show the color of the N-PTD before reaction with
a diene.
- To the second test tube add 15 drops of anthracene solution.
- To the third test tube add 15 drops of furan solution.
- Draw the solutions in test tubes 2 and 3 into a pipet in order to
mix the contents.
Hazards
Discussion
The Diels-Alder reaction was discovered in 1928 by German chemists Otto Diels and Kurt Alder, who received the Nobel Prize in chemistry in 1950 for their work towards understanding this 4+2 cycloaddition. The Diels-Alder reaction continues to be a widely used reaction in modern organic synthesis because in one step two carbon-carbon bonds are made in a stereoselective way.
In the Diels-Alder reaction, an alkene or an alkyne react with a conjugated diene to form an unsaturated six-membered ring. For this demonstration, a colorless diene (furan (1) or anthracene (2)) is reacted with a red dieneophile (4-phenyl-1,2,3-triazolin-3,5-dione (N-PTD) (3)) and a colorless adduct is formed.
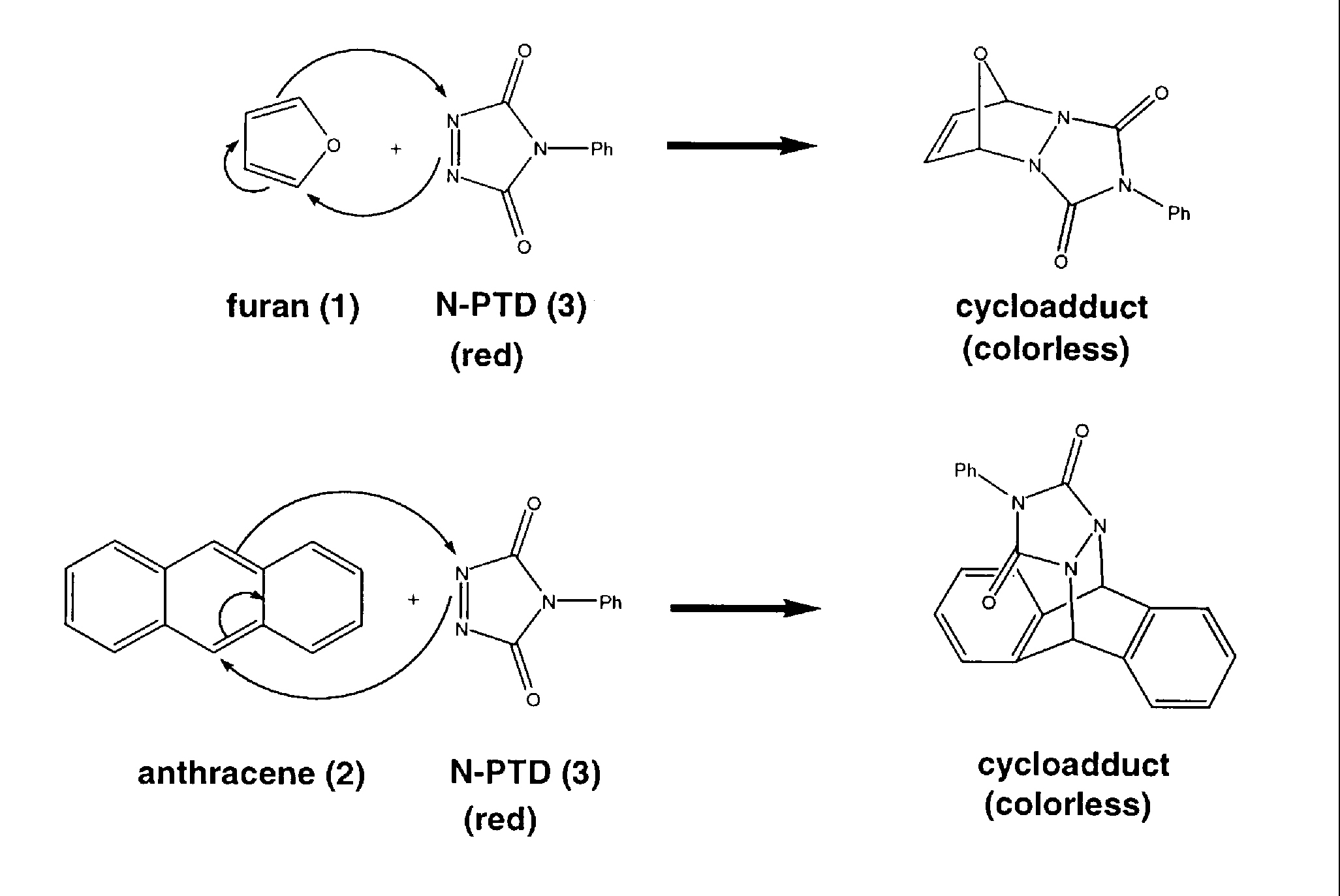
Click on any of the above thumbnails to open a window showing a larger image. Close the window and repeat to examine any of the other images. Close the window to continue with the demonstration information.
The idea for this demonstration and some of its procedures were taken from a website originating from the University of Regensburg1.
References
- http://www.uni-regensburg.de/Fakultaeten/nat_Fak_IV/Organische_Chemie/Didaktik/Keusch/p2_cyclo_add-e.htm (as seen on June 20, 2003)
Request a demonstration
The University of Minnesota is an equal opportunity educator and employer.
Copyright 2003 by the Regents of the University of Minnesota.
This page was last modified 7/11/2003.
For questions or comments, contact
Joseph Franek.






