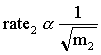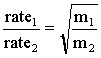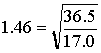Equipment
Specially constructed diffusion rate tube, laser pointer, ringstand, clamps, cotton swabs (2), small rubber bands (2).
Reagents
Concentrated HCl and NH3 solutions.
Presentation
- Clamp the glass tube to the ringstand so that it is exactly horizontal.
- Screw the two plugs, the small one holding the laser pointer and the larger one, into the appropriate threads on the glass tube.
- Adjust the clamp or rubber bands until the laser pointer comes on and stays on.
- Adjust the laser pointer orientation until it travels the length of the glass tube and strikes near the center of the large plug.
- Place a small rubber band on the shaft of each swab. Adjust the position of the rubberbands so that the head of the swab rests just above the main tube, so that the heads don't interfere in the laser beam path.
- Place several drops of concentrated HCl on one of the cotton swabs. Place several drops of concentrated NH3 on the second cotton swab.
- Place one swab into each of the access tubes located near the ends of the main tube. Allow the swabs to go down to the point that the rubber band on the shaft stops the travel.
- Dim the room lights.
- After about 3-4 minutes, the solid ammonium chloride particles will begin to form and they will scatter the laser light. This will produce a very visible red spot in the main tube where the particles have formed.
- You may measure the relative distances from the particle formation to the swab location. The results will conform reasonably well to Graham's law, or you may simply point out that the particles formed closer to the HCl swab than to the ammonia swab because ammonia is lighter and travels faster.
Hazards
Hydrochloric acid can irritate the skin. Hydrochloric acid vapors are extremely irritating to the eyes and respiratory system. Therefore, it should be handled only in well-ventilated area. Concentrated aqueous ammonia can cause burns and is irritating to the skin, eyes, and respiratory system. The light from laser pointers may cause eye damage if shown directly into the eye.
Discussion
The reaction we are examining is the following:
NH3(g) + HCl(g) ® NH4Cl(s)
The two gases are released at opposite ends of an enclosed glass tube. When the gases meet, they form ammonium chloride. The ammonium chloride is formed as a finely divided white powder, some of which remains suspended in the air and some of which deposits on the glass surface. Graham's law tells us that the rate of diffusion of a gas is inversely proportional to the square root of the molecular mass of the gas.
and
Given the molecular masses of HCl (36.5 g/mol) and NH3 (17.0 g/mol), we calculate a relative rate of
This says that in a given amount of time, NH3 should travel 1.46 times as far as HCl. In practice, we see the value of about 1.27. The reason for this is that the gases are actually diffusing through a third gas (air). There are additional collisions that retard the progress of the faster gas, NH3 to a greater degree than the slower gas, HCl. See [1] for more detailed treatment.
The first picture below shows the initial setup of the demonstration, the second picture show the scattered red laser light. The third picture show the same setup, but utilizing a green laser pointer and the fourth picture is a close up of the scattered green laser light, the lower streak is a reflection from the glass. If a green laser pointer is available, the scattered light is much more dramatic than with the red laser pointers.
 |
 |
 |
 |
Click on any of the above thumbnails to open a window showing a larger image. Close the window and repeat to examine any of the other images. Close the window to continue with the demonstration information.
References
- B. Z. Shakhashiri, Chemical Demonstrations, A Handbook for Teachers of Chemistry, Wisconsin, 1989, Vol.2, p.59-62.