Disappearing Color
Equipment
1 L or 500 mL Florence flask, stopper.
Reagents
1:3 ethanol:water solution with thymolphthalein indicator.
Presentation
- The solution should be a dark blue, if it is not blue, add 1 M NaOH until it is. This typically requires 1-1.5 mL per liter of solution.
- Fill a Florence flask approximately 1/2 to 1/3 full of the solution and stopper the solution.
- Have students unstopper the flask, whisper some command to the solution, restopper the flask and give it a gentle swirl.
- Have the student pass the flask to the next student.
- Repeat steps 3 and 4 until solution turns colorless.
- Return the solution to the storage bottle for use at a later date.
Hazards
Solid sodium hydroxide and concentrated solutions can cause severe burns to eyes, skin, and mucous membranes.
Ethyl alcohol is flammable.
Discussion
When this solution is exposed to a source of CO2, the pH will decrease and change the thymolphthalein indicator to its acidic form which is colorless. The ethanol keeps the thymolphthalein in solution. The following reactions illustrate what is occurring in the flask.
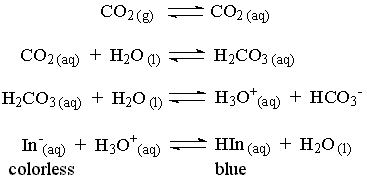
References
- L.R. Summerlin, J.L. Ealy, Chemical Demonstrations, A Sourcebook for Teachers, Washington D.C., American Chemical Society, Second Edition, 1988, Vol. 1, p.59.
Request a demonstration
The University of Minnesota is an equal opportunity educator and employer.
Copyright 2000 by the Regents of the University of Minnesota.
This page was last modified 3/26/2000.
For questions or comments, contact
Joseph Franek.