Genie in a Bottle
- Equipment
2 liter soda bottle (or other container -beaker, flask, etc),
goggles, spatula, mortar and pestle
- Reagents
30% hydrogen peroxide, manganese dioxide, it's catalytic effect
is greatest if it is freshly ground within 24 hours of use.
- Presentation
- Pour 50-100 mL of 30% hydrogen peroxide into 2 liter bottle (enough
to cover the "dimples") or container.
- Add a pea-sized amount of manganese dioxide to bottle or container.
- Gaseous oxygen will be emitted from the bottle. The reaction is exothermic; the soda bottle will get hot and shrink slightly.
- Hazards
30% hydrogen peroxide is a strong oxidizing agent; contact with eyes and skin should be avoided. In case of contact, flush with water for at least 15 minutes. Get medical attention if eyes are affected. Also avoid contact of hydrogen peroxide and combustible materials. 30% hydrogen peroxide must be stored in its original container.
- Discussion
Oxygen is a colorless, odorless gas at room temperature and atmospheric
pressure. The discovery of oxygen is typically attributed to Joseph Priestly.
However, it was Lavoisier who first realized this gas was a unique component
of air. Here oxygen is formed from the decomposition of hydrogen peroxide.
Hydrogen peroxide is not a very stable compound and its decomposition can
be induced by many means. Light will decompose it, which is why it is sold
in brown bottles in drugstores. The surface of manganese dioxide provides
a particularly favorable environment to catalyze the decomposition, though
the mechansim is somewhat poorly understood.
2 H2O2 (aq) 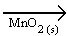 O2 (g) + 2 H2O(l)
O2 (g) + 2 H2O(l)
The "genie in the bottle" effect is finely divided water droplets propelled
from the bottle by the oxygen formed via the decomposition of hydrogen peroxide.

- References
- B. Z. Shakhashiri, Chemical Demonstrations, A Handbook for Teachers
of Chemistry, Wisconsin, 1989, Vol.2, p.137-141
Request a demonstration
The University of Minnesota is an equal opportunity educator and employer.
Copyright 2000 by the Regents of the University of Minnesota.
This page was last modified 03/02/2005.
For questions or comments, contact
Joseph Franek.
O2 (g) + 2 H2O(l)
