Magnetic Properties of Liquid Nitrogen and Liquid Oxygen
Equipment
Dewar flask or flasks capable of holding approximately 8 liters of liquid nitrogen, cryogenic gloves, 1-L Dewar flask fitted with a copper coil (see pictures), Rubber tubing to connect one end of the copper coil to the oxygen regulator and to direct the resulting liquid oxygen from the other end of the copper coil into a waiting Dewar flask, small Dewar flask, ringstand and ring to support the 1-L dewar flask, strong horn type magnet, insulated container large enough to contain magnet in a liquid nitrogen bath, wooden splint, insulating pad to protect counter top surface from the cold magnet. video camera for large classrooms.
Reagents
Approximately 8 liters of liquid nitrogen, tank of compressed oxygen with regulator assembly.
Preparation
- Carfully place the copper coil in the 1-L Dewar flask.
- Clamp the ring to the ringstand at a height just short of the height of the 1-L Dewar flask.
- Set the 1-L Dewar flask on the ringstand so that the ring supports the top of the flask.
- Connect length of rubber tubing to the ends of the copper coil of such length that they reach the oxygen regulator and the small Dewar flask. Clamp them if they feel loose.
- Fill the 1-L Dewar flask with liquid nitrogen.
- Adjust the oxygen regulator to an outlet pressure of approximately 10 p.s.i. and open the oxygen valve. The gaseous oxygen will be condensed to liquid oxygen as it flows through the copper coil which is held at the temperature of liquid nitrogen. You may have to refill the 1-L Dewar flask with liquid nitrogen in order to make enough liquid oxygen for the demonstration.
- Collect at least 25 mL of liquid oxygen in the small Dewar flask. Do not make a large excess of the liquid oxygen as it is a very hazardous material.
- At least 30 minutes before the demonstration, place the magnet in the insulated container and fill the container with liquid nitrogen. Cooling the magnet before the demonstration keeps the liquid oxygen and nitrogen from immediately vaporizing upon contact with the magnet.
Presentation
- Place the insulating pad on the counter, where you want the demonstration to take place.
- Wearing the cryogenic gloves, retrieve the magnet from its liquid nirogen bath and place it on the insulating pad.
- Pour a small amount of liquid nitrogen over the poles of the magnet. The liquid nitrogen should slide off of the poles without any hint of attraction.
- Pour a small amount of liquid oxygen over the poles of the magnet. The pale blue liquid oxygen will be held between the poles of the magnet. You can pass a wooden splint back and forth through the trapped liquid oxygen to prove that it is still liquid. The liquid oxygen will remain several minutes if the magnet has been sufficiently cooled beforehand. More liquid oxygen may be poured over the magnet poles if further study is desired,
Hazards
The high concentration of oxygen may result in the violent combustion of materials that would not normally be considered combustible. Liquid oxygen can be absorbed by clothing which leaves the clothing very susceptible to combustion for an extended period of time.
Both liquid nitrogen and liquid oxygen are very cold. Their boiling points are -196 oC and -183 oC respectively. Either of these liquids or any object cooled by them can cause severe frostbite.
Dewar flasks are subject to implosion. All Dewars should be covered with tape, netting or metal covers.
Compressed gas cylinders have the potential to become destructive missiles should their valves ever be broken. When in use, all cylinders should be securely fastened to a wall or cart.
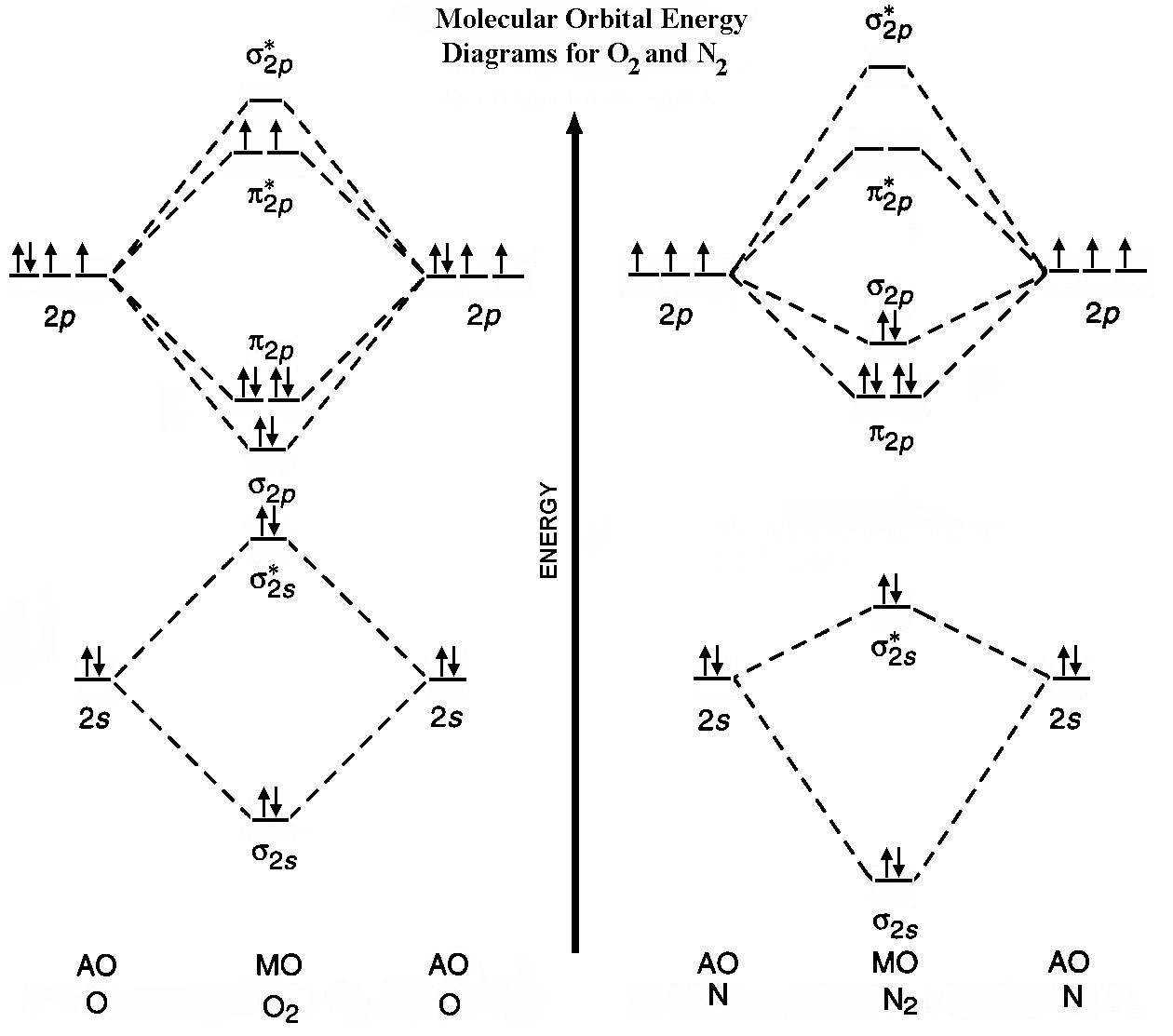
Discussion
References
- ???B. Z. Shakhashiri, Chemical Demonstrations, A Handbook for Teachers of Chemistry, Wisconsin, 1989, Vol.3, p.186-187
Request a demonstration
The University of Minnesota is an equal opportunity educator and employer.
Copyright 2000 by the Regents of the University of Minnesota.
This page was last modified 11/08/2002.
For questions or comments, contact
Joseph Franek.
